FDA Warning by Dr. Leonard Horowitz – Federal Drug Addicts – Fascist Regulatory Monopoly Enforcement

Inspecting The FDA’s Fascist Regulatory Monopolistic Enforcement Enterprise
or
How to Respond to an FDA Warning Letter
by
Leonard G. Horowitz, DMD, MA, MPH, DNM (hon.), DMM (hon.)
__________
Summary
Makers and promoters of natural health products have been defrauded. Many are jailed and killed. Health freedoms are denied everyone while the FDA-permits Big Pharma’s poisonings, “protection racketeering,” and subverting of the nation by corrupt justice department officials damaging the health and safety of every American.
Dr. Horowitz analyzes this catastrophe in the context of his personal “FDA Experience” responding to “Warning Letters” attempting to defraud him, to restrict his OxySilver advertising, and put him out of business. His analysis of the regulatory laws misrepresented by officials is monumental and central to any healthcare reform plan.
Neglecting this information is woefully damaging. Applying this information is wonderfully freeing. This is must reading for everyone praying for better health and healthcare as Trump appointees propose reforms.
Introduction
Most natural foods makers and users, especially persecuted and harassed natural healthcare professionals, know that the FDA punishes purported “violators.” Informed people also know there are serious conflicts of interest between FDA officials and the drug companies that poison people and the environment. “Regulatory capture” of the FDA, CDC and EPA by Big Pharma, Big Biotech, and Big Energy is at the root of what ails America. Widespread malfeasance in the Justice Department subservient to the drug makers and traffickers neglects law enforcement and regulatory rules amounting to organized crime in the monopolization of healthcare in violation of the Sherman Anti-Trust Act.
This condition of regulatory capture at the FDA damages American consumers otherwise benefited by low-to-no-cost and low-to-no-risk “natural” “alternatives” in healthcare. The suppression of natural beneficial products and services damages public health and the economy, and enslaves people to a criminal system of healthcare that produces widespread disease and mental illness through drug toxicity and vaccine side effects. Yet, the “little guys” making and prescribing “alternative medicines” are persecuted, publicized, indicted, convicted, and incarcerated.
This system is not just “broken.” The “swamp” requiring “draining” is not simply poisoned and genocidal. But the approach to reforming this monster from within the CULTure it creates is unreasonable and incapable of curing what is terminally ill.
This is a “case study” of the two major material misrepresentations of laws and regulatory powers abused by FDA officials keeping this healthcare fraud and genocidal crime in place.
The first misrepresentation confuses the nation by defying the intention of Congress in passing FDA drug regulations. It causes confusion over two simple words–“and” versus “or”–used in the most important law, 21 U.S.C. 321(g)(1). This misrepresentation and overstepped authority results in depriving citizens rights to free trade and free speech in the natural health products and services industries. This misrepresentation is so obvious, it must be intentional, and thereby is criminal, not excusable. This criminal confusion is administered to deprive citizens of truthful advertising to sell natural non-drug products, and has largely destroyed America’s drug-dependent economy and well-being of society.
The second blatant misrepresentation is made concerning the precise definition of the word “drug” that is central to the FDA’s regulatory powers. It is incredible, outrageous, and unconscionable that misrepresenting these three words–“and,” “or” and “drug” has sickened and enslaved America to the point of total collapse.
This article addresses each of these misrepresentations, and makes recommendations to the Trump Administration enabling product makers, advertisers, and users to make more informed decisions.(1)

Background
For more than a quarter century I have been active in the fields of public health, “alternative medicine,” and every aspect of natural health products commerce. I pioneered the “528LOVERevolution” featuring “medicinal music,” and co-developed the world’s first broad spectrum replacement for toxic vaccines and deadly antibiotics using the resonance frequency of 528nm/Hz. In addition, as an entrepreneur, I have brought many successful natural health products to market, and helped many doctors succeed in life-saving businesses. So when I learned that President Trump intends to “drain the swamp” in government, improve healthcare, and appoint a new FDA commissioner that may be Dr. Joseph Gulfo, I was all ears.
“We need to show the FDA love,” wrote Gulfo for Trump’s consideration. We need to “support FDA staff in doing their jobs and in the decisions they make.”
Dr. Gulfo’s additional proposals purportedly “better follow the intent of the law than the current system,” explained STAT online news magazine. For instance, Gulfo believes “the FDA should grant approval of some medicines even if they haven’t shown that they change patients’ health outcomes in the long term. . . . “
In other words, let’s trash common sense so long as it makes money, by fast-tracking unproven vaccines and drugs to enrich Big Pharma and Big Biotech investors.
“But Gulfo does not go as far as Jim O’Neill, a rival candidate for commissioner,” heralded the Boston-based Biotech-favoring publisher STAT. O’Neill “has advocated approving drugs when they’re proved safe, rather than testing them extensively for efficacy.”
Afraid O’Neill’s appointment might cut into STAT‘s commercial interests, the Big Biotech yellow press couldn’t help but add: “Gulfo dismisses [O’Neill’s] approach as selling ‘safe snake-oil.’”
With this sound-bite, I am deeply concerned about the concept of “draining the swamp” and then appointing people up to their eyeballs in the muck, like Dr. Gulfo. True, Gulfo has made fortunes developing numerous biopharma firms and medical technology enterprises. But those conflicting interests do not qualify him for making “America great again.” Based on what you are about to read, my case study in dealing directly with FDA regulators, we’d be far better off with O’Neill’s “safe snake-oil” than Gulfo’s profitable poisons.
Rather than treating FDA officials with “love,” they should be indicted for what they have done to the bodies, minds, and spirits of the American people in violation of anti-monopoly laws. The Administration has been complicit in genocide, and treason against the “Free World.”
FDA Warning Letters Help Administer Genocide
I document here my bias, conflicting interests, and “FDA experience” as a Harvard-trained expert in public health, author of numerous consumer health books, dozens of peer-reviewed scientific papers, and multiple award-winning documentary films critical of officials serving “Big Pharma,” including: In Lies We Trust: The CIA, Hollywood & Bioterrorism, PHARMAWHORES: The SHOWTIME Sting of Penn & Teller, and UN-VAXXED: A Docu-commentary for Robert De Niro.
 (CLICK HERE for one example.)
(CLICK HERE for one example.) Arguably, the FDA routinely sends warning letters to BigPharma companies too. A small set of such targets is shown by clicking the “CounterThink” comic below.
The FDA targets big companies as well as small ones. If only small companies like mine were targeted, then the public would instantly know the FDA or “Fraudulent Drug Advocates” were allied with groups such as the Partnership of New York City. This private commercial organization includes Lloyd Blankfein of Goldman Sachs (a major shareholder in AstraZeneca, implicated in both the H1N1 Flu Mist scandal and pandemic fraud, as well as the Halliburton explosion of the Transocean/BP oil rig in the Gulf.) By policing people like me for making honest claims based on published peer-reviewed science, as well as issuing occasional warnings and fines to large drug makers, the FDA appears legitimate.

Sadly, the FDA’s policies are overtly genocidal given the healthcare crisis in America and abroad. This is evidenced in many ways, including our abysmally high, third-world-like, infant mortality rate; our outrageous human sterility rates, obesity rates, hormonal-induction of homosexuality (i.e., “estrogenocide”) and more. . . . Clearly, the FDA permits and promotes, by their safety certifications, many deadly chemicals and even toxic wastes (like fluoride) to enter our water, foods, and consumer products from toothpastes to under arm deodorants. The government approves artificial sweeteners, antibiotics poisoning our poultry and water supplies, steroids in our cattle, mercury in our dental fillings and vaccines, and population-culling genetically-mutating viruses praised in our “immunizations.”
But little old me is alleged to risk the public’s health by supposedly making OxySilver “health claims” that FDA officials reserve for drugs. This practice clearly favors the drug industry’s monopoly over healthcare–arguably a “racket” protected by extortion in the form of such warning letters.
Once they claim, in a WARNING LETTER, that your product is a “drug” due to a legitimate health claim made in advertising, then the criminal branch of the “Fraudulent Deranged Aggressors” in the “Justice Department” pressures citizens to comply or be fined or jailed.
One example making news at the time of this writing is the case of UNITED STATES V. GIROD in the UNITED STATES DISTRICT COURT EASTERN DISTRICT OF KENTUCKY CENTRAL DIVISION (at Lexington), Criminal Action No. 5: 15-87-DCR (E.D. Ky. Jun. 24, 2016). Mr. Girod, clearly the victim here, is an Amish herbal salve maker convicted of, among other things, “obstructing government operations.”
News about Girod’s persecution, and others similarly situated, certainly produces more than public outrage. The “example” set by the prosecution, and such widely-publicized persecution, causes widespread fear across the nation, especially in the natural health products industry.
This industry, according to the nonprofit American Botanical Council (ABC), grossed more than $6.4 billion in 2014, and is increasing by more than 6% annually. The ABA’s market report is published in HerbalGram–a peer-reviewed quarterly journal. The “market share” enjoyed by naturalists represents less than two percent of the $425 billion annual sales of drugs.
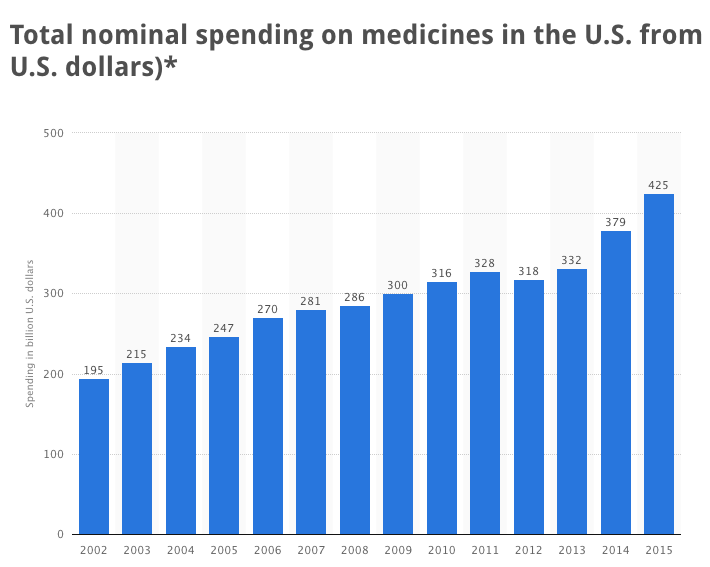 The correlation between such not-so-subtle extortion and society’s disproportionate reliance on drugs benefiting BigPharma is clearly criminal. By favoring Big Pharma using regulatory harassments, the FDA is literally aiding-and-abetting mass murder and omnicide, polluting our planet and poisoning virtually all life forms.
The correlation between such not-so-subtle extortion and society’s disproportionate reliance on drugs benefiting BigPharma is clearly criminal. By favoring Big Pharma using regulatory harassments, the FDA is literally aiding-and-abetting mass murder and omnicide, polluting our planet and poisoning virtually all life forms.
Controlled Opposition in Healthcare
Everyone raising anti-Big Pharma issues online, or promoting natural cures, get targeted by paid propagandists.
My Reply in Opposition to the FDA’s Warning Letter
Below is how I replied to the “Warning Letter” sent me by FDA and DHHS Officials Wagner, Autor, and Breen. My defenses serve as a model for others likewise extorted to reply to FDA warning letters. (Please feel free to distribute this writing liberally to help others similarly situated save lives and their businesses.)
To Whom It May Concern at the FDA:
I am responding to the ‘Warning Letter’ notice you mailed me on May 11, 2010, that states that you “have determined that [specified websites and products advertised] . . . are promoted for conditions that cause them to be drugs under section 201(g)(1) of the Federal Food, Drug, and Cosmetic Act (the Act) [21 U.S.C. $321(g)(1)].”
This section states: “(g)(1) The term “drug” means (A) articles recognized in the official United States Pharmacopoeia, official Homoeopathic Pharmacopoeia of the United States, or official National Formulary, or any supplement to any of them; and (B) articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in man or other animals; and (C) articles (other than food) intended to affect the structure or any function of the body of man or other animals; and (D) articles intended for use as a component of any article specified in clause (A), (B), or (C). A food or dietary supplement for which a claim, subject to sections 403(r)(1)(B) and 403(r)(3) or sections 403(r)(1)(B) and 403(r)(5)(D), is made in accordance with the requirements of section 403(r) is not a drug solely because the label or the labeling contains such a claim. A food, dietary ingredient, or dietary supplement for which a truthful and not misleading statement is made in accordance with section 403(r)(6) is not a drug under clause (C) solely because the label or the labeling contains such a statement . . .” [Emphasis added.]
Thus, kindly relay written evidence that any of our websites or products cited in your “Warning Letter,” are “recognized in the official United States Pharmacopoeia, official Homoeopathic Pharmacopoeia of the United States, or official National Formulary, or any supplement to any of them,” because my search has not found any.
Regarding the other concerns you raise in this “Warning Letter,” the legal interpretation of the word “and” in the aforementioned law, as per legal dictionaries means: “additionally” or “in addition to,” “together with,” or “along with;” not meaning, or misconstruing “and” to mean “or,” used to indicate an alternative, in this case the clauses (B), (C), and (D) referenced in 201(g)(1) [21 U.S.C. 321(g)(1)].
So as I always do my best to comply with laws governing commerce and proper labeling of products, I request from your legal department, or attorneys general handling such requests, a written statement specifically citing any law that requires any action by myself, or your department, neglecting clause (A); selectively requiring clauses (B), (C) and/or (D) as exclusively requiring compliance.
Thank you very much for consideration and timely response.
Sincerely yours,

Response to Director Breen
In response, Compliance Director Breen of the Seattle branch of the FDA replied as you can read by clicking this pdf file link:
FDA WARNING LETTER – May 27, 2010
Then, I replied to Director Breen through Compliance Officer Althar, as follows:
I am responding in good faith, and goodwill, in a timely fashion, with intent to comply with regulatory laws as written, and to Charles Breen’s letter of May 27, 2010, (received June 3, 2010) that responds to my question regarding 201(g)(1)(A)’s precise language pertaining to the use of the conjunctive “. . . ; and . . .”
Director Breen wrote on May 27, 2010, that “the plain meaning of the statute is that the product is a “drug” under the Act if it meets any one of those four subclauses.” Yet he neglected to respond to my request for anything in writing, from an attorney general preferably, that would provide tangible written evidence that the U.S. Congress had intended the broad interpretation of this conjunction, as the FDA, and your office, is currently demanding.
Director Breen’s letter also threatens my person and property with, “enforcement action without further notice,” if I do not respond to your office “within fifteen (15) working days of the receipt of the Warning Letter.” Thus, this response is timely; and designed to prevent armed FDA agents, and complicit law enforcement officials, from invading my office (as has been done in the past to some of my colleagues), confiscating property, products, computers and records, etc., and initiating lengthy and costly criminal proceedings.
Thus, I take this matter very seriously, as do you, and I expect that you will, likewise, comply with the reasonable respectable requests that I make to serve this lawful process.
Compliance Plan and Need for Further Discovery
As I intend to fully comply with the law, my compliance PLAN of action includes the following:
1) Review and determination of statutory requirements necessary to confirm violations of law, false claims, or improper labeling; followed by
2) Rewording, relabeling, and/or clarifying text contained on websites and advertisements in violation of laws, improper label text, or false or misleading claims; or
3) Requesting and receiving from the FDA appropriate disclaimers for each qualified claim necessary, identified, and legitimized by published science; followed by
4) Posting FDA official disclaimers where warranted to remedy grievance(s).
To begin this process as detailed above, as previously written (May 17, 2010), I need to confirm any of our advertisements violate 201(g)(1) of the Federal Food, Drug, and Cosmetic Act (the Act) [21 U.S.C. $321(g)(1)], because the words “and” and “or” have two distinct meanings in common usage and law; and the word “and” is used between (g)(1)(A) and (B) of this statute, thus exempting products advertised that are not cited in “articles recognized in the official United States Pharmacopoeia, official Homoeopathic Pharmacopoeia of the United States, or official National Formulary, or any supplement to any of them; . . .”

As a compliance officer for the FDA, you should have available to you some writing that legitimizes the meaning Director Breen has provided me; otherwise, a legal review of US Congressional hearings and records on this matter is in order at this time in which I, and the FDA, is under increasing scrutiny. You and I are both aware that the FDA risks civil and criminal complaints for overstepping its authority, as published in the May 7, 2010, issue of FDA News (Vol. 7: No. 90; [Volume subsequently removed from Internet]).(6)
Thus, I consulted a couple of licensed attorneys in an effort to gain more clarity and certainty in this matter. I also reviewed law reviews pertaining to the legitimate interpretation of the use of the word “and,” and adjacent semicolon “;” punctuation, coupled with the conjunctive.
The attorneys have advised me that they are aware that the FDA routinely assumes Director Breen’s position, yet they were unsure this assumption is a valid interpretation of the intention of Congress in enacting this law; and I have been advised that the interpretation, and pending enforcement action(s), may be unlawful under US Constitutional First Amendment rights.
 In fact, in Thompson v. Western States Medical Center, 535 U.S. 357 (2002), the Supreme Court was confronted with a FDA case involving abridgement of First Amendment rights. This case involved some compounding pharmacists who challenged provisions of the FDCA that restricted their advertising. In finding the advertising restriction violative of the First Amendment, the Supreme Court articulated a specific test: if commercial speech relates to unlawful activity, or is misleading, then it lacks First Amendment protection. But if the commercial speech is not related to illegal activity nor is it misleading, then there must be a substantial governmental interest in regulating the speech. “If the First Amendment means anything, it means that regulating speech must be a last — not first — resort.” /Id./, at 373. The government cannot prevent “the dissemination of truthful commercial information in order to prevent members of the public from making bad decisions with the information.” /Id./, at 374.
In fact, in Thompson v. Western States Medical Center, 535 U.S. 357 (2002), the Supreme Court was confronted with a FDA case involving abridgement of First Amendment rights. This case involved some compounding pharmacists who challenged provisions of the FDCA that restricted their advertising. In finding the advertising restriction violative of the First Amendment, the Supreme Court articulated a specific test: if commercial speech relates to unlawful activity, or is misleading, then it lacks First Amendment protection. But if the commercial speech is not related to illegal activity nor is it misleading, then there must be a substantial governmental interest in regulating the speech. “If the First Amendment means anything, it means that regulating speech must be a last — not first — resort.” /Id./, at 373. The government cannot prevent “the dissemination of truthful commercial information in order to prevent members of the public from making bad decisions with the information.” /Id./, at 374.Now there is nothing unlawful about our activity, or misleading about the advertisements that you claim makes our products “drugs.” Some of our products may be insufficiently labeled, and I seek to remedy this possible deficiency during the course of these compliance proceedings.
I also found the following confirmation pertaining to the substantive material use of this “; and” conjunction (in the 201g(1) regulation) that appears to contradict Director Breen’s assumption. This is from: A Law Dictionary, Adapted to the Constitution and Laws of the United States. By John Bouvier. Published 1856, Vide Toull. liv. 3, t. 2, c. 5, n. 430; 4 T. R. 65; Barringt. on the Stat. 394, n. Vide article Points, regarding the semi-colon punctuation’s use in legal instruments:
“All such instruments are to be construed without any regard to the punctuation; and in a case of doubt, they ought to be construed in such a manner that they may have some effect, rather than in one in which they would be nugatory.”
Thus, I must insist on a written clarification, by your attorneys general. They should provide me, in writing in a timely fashion, how and why the FDA chooses, at this time, in my case, to directly disregard the meaning, and intended meaning, of the word “and.”

Otherwise, I must assume by Director Breen’s threatening letter, and disregard to this, my kindly second, request for material evidence supportive to the FDA claims against me, that you are administering a hostile un-American action on behalf of a fascist pro-pharmaceutical regime, applying harassment, and unwarranted aggression against me, damaging to my person, properties, and business interests; all consistent with organized crime and pharmaceutical racketeering.
Also, if you fail to reply to this demand for more information within 15 working days, to facilitate my timely compliance with FDA demands, then you may be held accountable for damages, and make null and void previous FDA Warning Letter and Dir. Breen’s notice.
Implementing Compliance Plan, Step 1: Ombudsman’s Clarification
Regarding Step 1 of my compliance program, if you are unable to provide me with an official Congressional, or Agency writing authorizing the FDA’s broad interpretation of this “; and” conjuction, and based on this, your agency insists that I come into compliance with a law that I may not have broken, or does not apply to my circumstance, then I demand a legal review of this matter from the FDA’s Ombudsman’s office as the next step.
Thus, please pass this request for the Ombudsman’s review of the US Congress’s intent in the wording of 201(g)(1)(A) that includes the precise reason any reasonable person would interpret the word “and” to mean “or,” and why the FDA is not overstepping its authority in assuming the assumption Dir. Breen provided is reasonable and constitutionally valid.
Implementing Step 2
Assuming you are positioned to advance an armed attack against my person and premises, and that you intend to do so promptly, as Director Breen has threatened, then under protest, with this NOTICE becoming part of my legal record, I propose to administer in the next 30-days:

a)Censoring every word that you, or your FDA officers, have found objectionable using the following phrase, “FDA Censored Word*.” I propose replacing words in our advertisements and website text pertaining to “articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in man” with “FDA Censored Word*.” The asterisk would indicate reference to a footnote to include either: 1) the set of censored words that the FDA finds objectionable, with an explanation why the FDA finds these words objectionable, pertaining to its policy (allegedly intended) to protect consumers and the public’s health; or 2) a simple statement pertaining to the FDA’s policy, written by you or another FDA official, stating why it has censored certain words and statements, and/or finds them objectionable.
b)Adding the following NOTICE, and official company disclaimer, to every page of our websites containing text the FDA finds objectionable. The following notice uses OxySilver as an example, but can be easily modified for other products referenced in Director Breen’s Warning Letter of May 11, 2010:
WARNING: DO NOT BUY OXYSILVER, the first and only silver hydrosol exclusively pioneering hydrosonically-charged covalently-bonded oxysilver molecules energetically delivering optimal purgative and restorative value, if you give credence to the Food and Drug Administration that objects to us informing you about the potentially miraculous benefits that may result from taking OXYSILVER™ as recommended. This notice and disclaimer regards recently passed FDA regulations, section 201(g)(1)(B) of the Federal Food, Drug, and Cosmetic Act, and the dangerous deceptive Act itself [21 U.S.C. § 321(g)(1)(B) defining a “drug” as ANYTHING said, written, or claimed to “cure, mitigate, treat, or prevent” any disease, ailment, or illness. Sunlight is a “drug” under these laws for curing infantile jaundice as per claims written in medical textbooks. BE FULLY INFORMED AND WARNED: OXYSILVER™ is not a drug, regardless of what the FDA and these laws say; as clearly the word “DRUG,” like the word “immunization,” has been redefined in an organized criminal conspiracy to enable the pharmaceutical industry to control natural healing methods, materials, their manufacturers, distributors, and consumers; “DRUG” is hereby responsibly decreed to mean, “Degenerate Regulators Undermining Go(o)d” as evidenced by the FDA’s violation of Federal RICO laws section 901(a) of the Organized Crime Control Act of 1970 (Pub.L. 91-452, 84 Stat. 922, enacted 1970-10-15), and Chapter 96 of Title 18 of the United States Code, 18 U.S.C. § 1961–1968, as well as the documented despicable effect of FDA-approved drugs—the leading causes of morbidity and mortality in the United States according to published medical statistics. Notice is hereby given: Any reference to OXYSILVER™, curing, mitigating, treating, or preventing any disease shall be construed as legal and lawful notice of qualified claims made properly substantiated by solid scientific evidence.
Implementing Step 3:
Now assuming we cooperatively advance my compliance plan as stated, your administration is required to acknowledge the legitimacy and legality of qualified claims, that I believe, at this present time, we have made, as listed in your WARNING LETTER of May 11, 2010.
I am aware that your authority requires you, in service to the American people and your commission, to supply me with appropriate disclaimers for posting where necessary, as per a US District Court For The District of Columbia decision (May 27, 2010; Alliance for Natural Health US, et al., v Kathleen Sebelius, et al; Civ. No. 09-01470 (ESH)) wherein the Court remanded to the FDA proposed claims “for the purpose of 1) drafting one or more disclaimers to accompany plaintiffs’ . . . claims, or, alternatively, setting forth empirical evidence that any disclaimer would fail to correct the claims’ purported misleadingness . . .”

Thus, qualified claims based on solid published science are required, and the FDA, in its regulatory capacity, is required to compose one or more disclaimers for every alleged violation of law it seeks to remedy, or provide “empirical evidence that any disclaimer would fail to correct the claims’ purported misleadingness.”
So providing we can resolve the first substantive matter of statutory language regarding the intended meaning of “and” versus “or,” requiring some kind of written legal opinion from the FDA’s Ombudsman’s office initially, subject to appeal, then I pledge to advance, in a timely manner, remedies to specific FDA objections, step by step, item by item, as referenced in Director Breen’s WARNING LETTER of May 11, 2010.
For example, in reference to the FDA’s objection to the phrases, “Destroy viruses, Bacteria, and Fungi,” “Eliminate the need for harmful vaccines and antibiotics,” and “Silver hydrosols, in general, are superior powerful broad spectrum anti-microbials,” qualified claims provided by the FDA will be posted that:
1) Shall reference the mountain of science, as per the University of Wisconsin’s nano-silver science literature review freely available online here:http://www.nanoceo.net/nanorisks/silver-particles, that proves silver hydrosols, as a general class of products, destroy viruses, bacteria, and fungi; eliminate the need for harmful vaccines and antibiotics, and are, in general, superior powerful broad spectrum antimicrobials.”

2) Link the FDA’s objections, and/or censorship of the words “vaccines and antibiotics” to the statement regarding OxySilver as an alternative to such “harmful vaccines and antibiotics,” wiith a qualified claim referencing the FDA’s cooperative study with the Merck drug company linking the origin of HIV/AIDS from chimpanzee cultured hepatitis B vaccines given, 1970-1974, to gay men in NYC, Willowbrook State School mentally retarded children on Staten Island, NY, and central African villagers, as scientifically detailed and referenced by Purcell (See: Purcell RH. Current understanding of hepatitis B virus infection and its implications for immunoprophylaxis. In: Antiviral Mechanisms: Perspectives in Virology IX. The Gustav Stern Symposium. New York: Academic Press, 1975 pp. 49-76.)
Furthermore, I propose constructing a website, linked from all of the websites cited in the Warning Letter that I administer, that displays the FDA’s Warning Letter(s), communications, and our interrogatories for optimal transparency, service to our customers and for the benefit of the general public.
 In closing, and pledging my goodwill in this public service and regulatory process, I assume we are both pledged to act in the best interest of public health and safety. In honoring transparency, and what is obvious and substantive to these proceedings, the FDA and your office is being scrutinized at this time, for gross conflicting pharmaceutical interests, disserving, and genocidally impacting, the American people.
In closing, and pledging my goodwill in this public service and regulatory process, I assume we are both pledged to act in the best interest of public health and safety. In honoring transparency, and what is obvious and substantive to these proceedings, the FDA and your office is being scrutinized at this time, for gross conflicting pharmaceutical interests, disserving, and genocidally impacting, the American people.I pray that our collaboration and remediation of issues raised herein shall serve, far beyond our offices, the general good.
Thank you very much, in advance, for your cooperation and timely response.
Sincerely yours,
Healthy World Distributing, LLC
By: Leonard G. Horowitz, Overseer, Managing Member
and
Healing Celebrations, LLC
By: Leonard G. Horowitz, Overseer, Managing Member
– End of response letter –
First Amendment Violations
Contrary to the evidence above showing FDA agent Breen blatantly misrepresented the plain definition and meaning of the word “drug” in his May 27, 2010 letter, FDA officials and agents are supposed to be well-trained, competent, and ethical in their conduct; free from conflicting interests, as evidenced by Title 21, Chapter 1, Subchapter A, Section 19.6 of their Code of Ethics.
What is frightening, however, in violation of First Amendment due process rights, there appears to be a “loophole” in the code that permits agents to omit the notice-mailing “due process” requirement affirmed by courts, as in Alliance for Natural Health US, et al., v Kathleen Sebelius (Id.)
It appears from the Code that the FDA, in some circumstances, does not need to Notice alleged criminals to obtain a response prior to invading the suspect’s privacy and rights, according to: §7.84 Opportunity for presentation of views before report of criminal violation. Although it is unethical to deprive suspects of their Constitutional rights, and such deprivation may be actionable and counterclaimed, it is conceivable that the “Fascist Drug Addicts” appear authorized to commit un-American activities in violation of their duty to up-hold the Constitution.
Obfuscating and Misrepresenting Natural Cures as “Drugs”
(3) Key Definitions of legal terms used by the FDA are published HERE.
|
Title 21 → Chapter I → Subchapter A → Part 3 → Subpart A → §3.2 |
Evidencing intent to confuse the public, and “stonewall” victims into submission, the FDA’s provision of its definition of “DRUG” is not easy to locate. It appears to be concealed within a labyrinth of other regulations as shown by this statement in the General Provisions:
“(g) Drug has the meaning given the term in section 201(g)(1) of the act.”
Then, that reference states the following HERE:
“a) A drug or drug product (as defined in 320.1 of this chapter)”
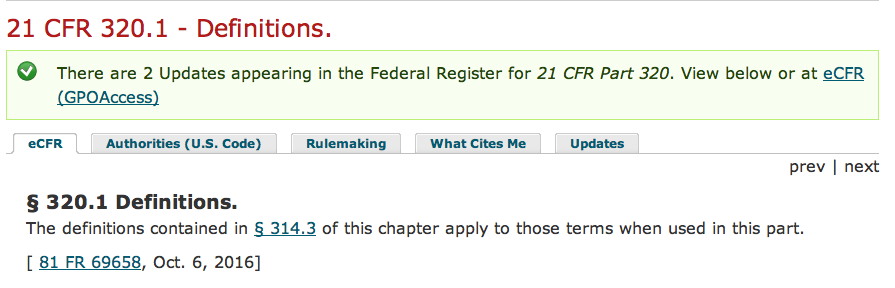
And if that isn’t confounding enough, further obfuscating and redirecting occurs in the text provided by 320.1:
The definitions contained in § 314.3 of this chapter apply to those terms when used in this part.”
 Here, the reference to § 314.3 does NOT actually define the word “DRUG.” It defines the word “drug substance” only, as shown in this screenshot:
Here, the reference to § 314.3 does NOT actually define the word “DRUG.” It defines the word “drug substance” only, as shown in this screenshot:
 This definition of “drug substance” appears to be what FDA “Warning Letters” misrepresent as cause to make false claims against criminal suspects–that is, every natural food and natural health product manufacturer. This definition states:
This definition of “drug substance” appears to be what FDA “Warning Letters” misrepresent as cause to make false claims against criminal suspects–that is, every natural food and natural health product manufacturer. This definition states:
“Drug substance is an active ingredient that is intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or any function of the human body, but does not include intermediates used in the synthesis of such ingredient.”
Key words in this definition include: “active ingredient,” “intended,” and “pharmacological activity.”
What is an “Active Ingredient” by Law?
The “active ingredient” link provided by § 314.3 states:
“Active ingredient is any component that is intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease, or to affect the structure or any function of the body of man or other animals. The term includes those components that may undergo chemical change in the manufacture of the drug product and be present in the drug product in a modified form intended to furnish the specified activity or effect.”
There are several legal problems with this definition of “Active ingredient,” beginning with the definition of “pharmacological activity.” Curiously, this is NOT defined in any section of FDA code, according to this study. This omission evidences “foul play” and “bad faith,” because it is unreasonable to presume lawmakers and FDA officials, in crafting thousands of pages of related code, would have “accidentally” omitted this most important definition by “excusable neglect.”
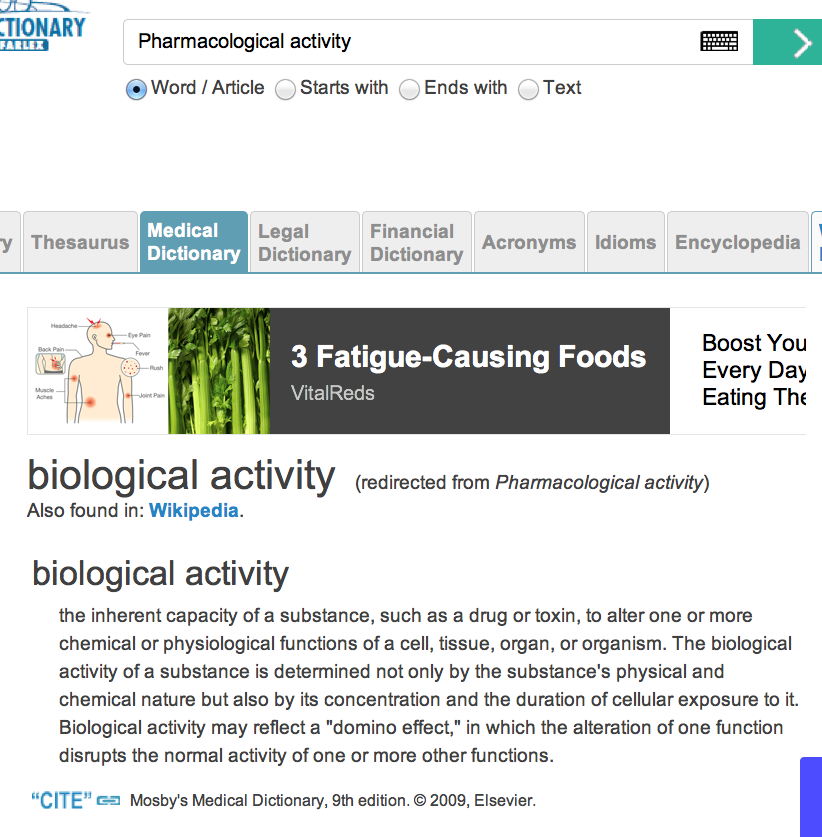
To define “Active ingredient,” it requires a fact finder to reference a medical dictionary. Here is where the “smoke and mirrors” game gets a lot worse. “Pharmacological activity” is not defined in online references. For instance, the Free Online Medical Dictionary, that cites Mosby’s Medical Dictionary, 9th edition. © 2009, Elsevier, omits this all important definition. Elsevier’s definition redirects to “biological activity,” as proven by this screenshot:
 Further researching in the meanings of “pharmacological activitiy” and “active ingredient” clearly gives the impression of intentional industry-wide fraud. The entire matter of claiming the FDA code validly regulates natural medicines such as herbs, tinctures, homeopathics, etc. is controverted by this research. FDA REGULATION OF THE NATURAL FOODS AND HEALTH PRODUCTS INDUSTRY IS BASELESS, AND A COMPLETE CONSUMER FRAUD AND REGULATORY RACKET, ACCORDING TO THIS THOROUGH AND REASONABLE ANALYSIS OF THE DEFINITIONS IN LAW AND MEDICINE.
Further researching in the meanings of “pharmacological activitiy” and “active ingredient” clearly gives the impression of intentional industry-wide fraud. The entire matter of claiming the FDA code validly regulates natural medicines such as herbs, tinctures, homeopathics, etc. is controverted by this research. FDA REGULATION OF THE NATURAL FOODS AND HEALTH PRODUCTS INDUSTRY IS BASELESS, AND A COMPLETE CONSUMER FRAUD AND REGULATORY RACKET, ACCORDING TO THIS THOROUGH AND REASONABLE ANALYSIS OF THE DEFINITIONS IN LAW AND MEDICINE.
Further inquiry into the term “pharmacological” returns results such as this screenshot, again from the online medical dictionary.
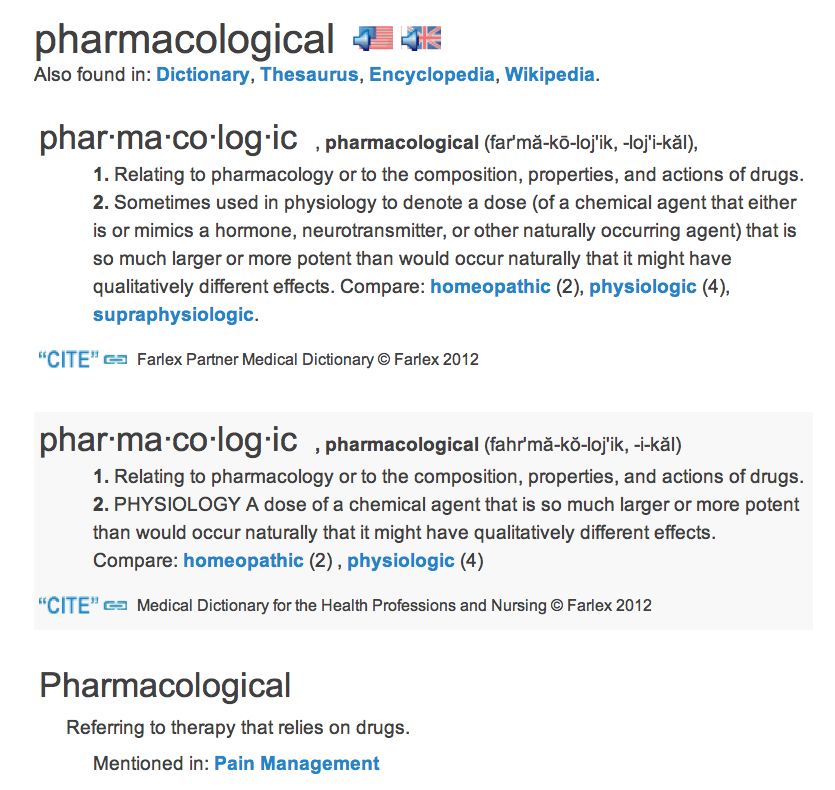 From this definition, it is clear that the DEFINITION STRICTLY AND EXCLUSIVELY APPLIES TO DRUG MAKERS IN THE DRUG INDUSTRY, and certainly not the natural healing industry.
From this definition, it is clear that the DEFINITION STRICTLY AND EXCLUSIVELY APPLIES TO DRUG MAKERS IN THE DRUG INDUSTRY, and certainly not the natural healing industry.
What is the “Intent” to Defraud the Public and Who is Really Guilty?
The word “intended” in § 314.3 is of utmost importance in law, and in this regulatory code.
Here is the sentence again with emphasis added. . . . “Active ingredient is any component that is intended to furnish pharmacological activity or other direct effect . . .”
That expressly means that natural products makers must have the conscious intent to make a drug, furnish a pharmacological activity, or intent to produce a product that has a direct effect (not a non-specific or general immunity-boosting impact) “in the diagnosis, cure, mitigation, treatment, or prevention of disease, or to [intend to directly] affect the structure or any function of the body of man or other animals.
Accordingly, that leaves out all natural anti-microbials (such as OxySilver, garlic remedies, anti-viral vitamin C, and anti-microbial essential oils) since microbes are not humans “or other animals.”
And generically defending the natural health products industry, discussion by the Supreme Court in Ernst & Ernst v. Hochfelder, 425 US 185, 201 – Supreme Court 1976, gives pause to consider who the real demons are.
Was it the intention of Congress to target ma and pa small businesses making natural remedies using herbs and nutrients in their kitchens, labs, or barns, as the FDA targets here, or was it the intention of lawmakers to regulate drug-makers expressly?
The Supreme Court (Id) offers insight pursuant to FDA officials acting contrary to a reasonable grasp of their legislative authority:
“It is difficult to believe that any lawyer, legislative draftsman, or legislator would use these words [especially the word “intended” in § 314.3] if the intent was to create liability for merely negligent acts or omissions.[23]”
In other words, those guilty of criminal activity must consciously intend to defraud the public, or purposely intend to cause harm, to qualify for FDA interrogation and prosecution.
Congress cannot be deemed to have intended to punish anyone who is not “plainly and unmistakably” within the confines of the statute. United States v. Lacher, 134 U.S. 624, 628; United States v. Gradwell, 243 U.S. 476, 485.
Quoting Judge Marshall in Dotterweich (at 280) :
” . . . § 303 (c). . . . affords immunity from prosecution if certain conditions are satisfied. The condition relevant to this case is a guaranty from the seller of the innocence of his product. So far as here relevant, the provision for an immunizing guaranty is as follows:
“No person shall be subject to the penalties of subsection (a) of this section . . . (2) for having violated section 301 (a) or (d), if he establishes a guaranty or undertaking signed by, and containing the name and address of, the person residing in the United States from whom he received in good faith the article, to the effect, in case of an alleged violation of section 301 (a), that such article is not adulterated or misbranded, within the meaning of this Act, designating this Act . . .
“The prosecution to which Dotterweich was subjected is based on a now familiar type of legislation whereby penalties serve as effective means of regulation. Such legislation dispenses with the conventional requirement for criminal conduct — awareness of some wrongdoing. In the interest of the larger good it puts the burden of acting at hazard upon a person otherwise innocent but standing in responsible relation to a public danger. United States v. Balint, 258 U.S. 250. And so it is clear that shipments like those now in issue are “punished by the statute if the article is misbranded [or adulterated], and that the article may be misbranded [or adulterated] without any conscious fraud at all. . . .”
So even assuming a natural product maker neglected or omitted ingredients or disclaimers on the label, the Congress did not draft this regulatory code to charge guiltless citizens with crimes. They only demanded punishment of anyone who “misbranded [or adulterated] the product or its labeled, whether intending to do so consciously or not. The FDA has used this fact to overstep its authority by first claiming non-drugs are drugs, and then claiming natural product labels are misbranded.
But the clear reading of § 314.3 and Dotterweich, and the facts surrounding many FDA prosecutions, shows the proverbial “shoe is on the other foot.” The FDA is intentionally “misbranding” violators by omitting, neglecting, and misrepresenting common and statutory laws. Administrators are intentionally overstepping their authority and victimizing an entire industry. This can be known because they are not stupid people. They can read the code and case law, and the aforementioned definitions as I have. Instead they act as outlaws with “scienter”–or conscious intention (not simple negligence) to break their own laws.
They also issue frivolous and fraudulent “Warning Letters” to non-offenders, and output press releases heralded by newspapers and broadcasters frightening consumers and manufacturers using the aforementioned misrepresentations and false presumptions.
It is unreasonable to conclude otherwise. The § 314.3 law, its words, language, and intent of Congress is clear; and is clearly being misrepresented by FDA agents such as Breen.
The Supreme Court in Ernst & Ernst v. Hochfelder, (Op. cit.) went on to state, “Neither the legislative history nor the briefs supporting respondents identify any usage or authority for construing “manipulative [or cunning] devices” to include negligence.[24]”
Applying this wisdom to FDA officials’ actions as regulators in the natural health products industry, these agents are not authorized to target citizens who are simply intending and acting in good faith to produce good quality pure consumer products to improve and sustain health in humans and animals, while not “misbranding” or “adultering” ingredients on labels.
Officials are acting contrary to law when issuing Warning Letters (like the ones attached hereto), exceeding their authority, since this can be reasonably construed as ““manipulative [or cunning] devices” to defraud manufacturers, consumers, and monopolize healthcare, in favor of Big Pharma.
These facts, and FDA’s deeds, evidence gross actionable negligence and liability in tort law for all the pain, suffering, and financial damages these outlaws have caused.
President Trump has pledged to “drain the swamp” within regulatory agencies while advancing a healthcare plan emphasizing prevention and self-care. These facts encourage “swamp draining” at the FDA most urgently.
Opposing FDA Railroading of Victims
The preceding information has great practical and legal implications in public health.
For instance, the case of the Kentucky Amish herbalist, Sam Girod, persecuted and prosecuted by federal officials in the case of UNITED STATES V. GIROD in the UNITED STATES DISTRICT COURT EASTERN DISTRICT OF KENTUCKY CENTRAL DIVISION (at Lexington), Criminal Action No. 5: 15-87-DCR (E.D. Ky. Jun. 24, 2016) requires reconsideration.
Under Kentucky criminal code, and federal criminal code, a retrial or reversal may be obtained due to the aforementioned newly obtained evidence of FDA fraud, in favor of Girod. The federal rule states:
Rule 33. New Trial
(a) Defendant’s Motion. Upon the defendant’s motion, the court may vacate any judgment and grant a new trial if the interest of justice so requires. If the case was tried without a jury, the court may take additional testimony and enter a new judgment.
(b) Time to File.
(1) Newly Discovered Evidence. Any motion for a new trial grounded on newly discovered evidence must be filed within 3 years after the verdict or finding of guilty. If an appeal is pending, the court may not grant a motion for a new trial until the appellate court remands the case.
Relevant case law, as aforementioned, is supplemented by Brady v. Maryland, 373 US 83 – Supreme Court 1963. That case emphasized necessary due process while reconsidering the prosecution’s “suppression of evidence favorable” to the accused as a denial of due process. 195 F. 2d, at 820. In Napue v.Illinois, 360 U. S. 264, 269, . . . we said: “The same result obtains when . . . false evidence” is permitted to go “uncorrected when it appears.” And see Alcorta v.Texas, 355 U. S. 28; Wilde v. Wyoming, 362 U. S. 607. Cf. Durley v. Mayo, 351 U. S. 277, 285 (dissenting opinion).
“Fraud” means an intentional misrepresentation, deceit, or concealment of a material fact known to the [FDA in this case as] defendant with the intention on the part of the defendant of thereby depriving a person of property or legal rights or otherwise causing injury. In re First Alliance Mortgage Company, 471 F. 3d 977 – Court of Appeals, 9th Circuit 2006,
By suppressing the actual definition of “drug”, and misrepresenting violators, while making it extremely difficult for a reasonable citizen to discern what is fraudulent in the malicious prosecution of FDA targets, as we see with Mr. Girod, a mistrial for the prosecutors’ fraud should not go unchallenged.
In a critical commentary favoring fast tracking drugs, Thomson Reuters published in BioWorld about the FDA:
“Its system of warning letters provides no formal or timely system of appeal, while its rules on off-label promotion have always played fast and loose with the First Amendment. In short, the agency commonly takes a “by any means necessary” approach to fulfilling its mission without strict regard to statute.”
As a person and public health expert repeatedly targeted by the FDA, I conclude that Big Pharma’s capture of this government entity extends a virtual monopoly over healthcare control beyond evaluating food and drug safety and efficacy. Contrary to Congress’s intent, the FDA is aiding-and-abetting genocide. It suppresses natural cures, reduces access to remedies, and discourages distribution and dispensing of helpful products to doctors, pharmacists, and society. The public, by members of the press and FDA’s press corps, has been intentionally deceived to erroneously presume the FDA acts in good faith. Nothing can be farther from the truth in the Administration’s advocacy of drugs and vaccines.
___________________
(1) This article is not intended to serve as a substitute for competent legal counsel. The author is not a licensed lawyer. The author does, however, encourage pro se litigation when necessary to defend civil rights and property rights. He highly recommends ProSeLegalAide.com as extremely helpful for this purpose.
 _______________________________
_______________________________
 About the author: Dr. Leonard Horowitz is the author of nineteen books including three American best-sellers, Emerging Viruses: AIDS & Ebola–Nature, Accident or Intentional?, Healing Codes for the Biological Apocalypse, and Healing Celebrations: Miraculous Recoveries Through Ancient Scripture, Natural Medicine and Modern Science. The doctor’s The Book of 528: Prosperity Key of Love, is currently revolutionizing the music industry and natural healthcare. Dr. Horowitz had been advancing an alternative to the duplicitous World Health Organization (WHO) until agents for Big Pharma’s protection racket libeled and cyber-bullied his good work, and smeared his reputation using the FDA Warning Letters attached hereto. Consequently, the doctor believes it is his public duty to expose the fraud and crime damaging consumers and others like him in the natural healing arts and sciences.
About the author: Dr. Leonard Horowitz is the author of nineteen books including three American best-sellers, Emerging Viruses: AIDS & Ebola–Nature, Accident or Intentional?, Healing Codes for the Biological Apocalypse, and Healing Celebrations: Miraculous Recoveries Through Ancient Scripture, Natural Medicine and Modern Science. The doctor’s The Book of 528: Prosperity Key of Love, is currently revolutionizing the music industry and natural healthcare. Dr. Horowitz had been advancing an alternative to the duplicitous World Health Organization (WHO) until agents for Big Pharma’s protection racket libeled and cyber-bullied his good work, and smeared his reputation using the FDA Warning Letters attached hereto. Consequently, the doctor believes it is his public duty to expose the fraud and crime damaging consumers and others like him in the natural healing arts and sciences.




