Influence of various intensities of 528Hz sound-wave in production of testosterone in rat’s brain and analysis of behavioral changes
Genes & Genomics volume 41, pages201–211(2019)
Abstract
Testosterone is a nuclear androgen receptor ligand that controls multiple pathways in brain. In addition to the active biosynthesis of steroids in classic steroidogenic organs such as gonads, adrenals and placenta, testosterone also produced in astrocyte cells of brain. Testosterone and its level must be regulated in brain; because, it directly and indirectly affects memory and several key behavioral characteristics. The significance of sound waves on key enzymes that regulate levels of testosterone in brain has not been investigated. The aim of our study was to examine physical stress of such as sound on induction behavioral changes in animal models. According to the current study, sound waves with 528Hz frequency in 100 dB intensity induce testosterone production in brain by enhancing StAR and SF-1 and reducing P450 aromatase gene expression. Frequency of 528 Hz also reduces total concentration of reactive oxidative species in brain tissue. Prolonged exposure to this sound wave showed reduction of anxiety related behaviors in rats. The results reveal that reduced anxiety is related to increased concentration of testosterone in brain. This study may lead to ascertain a possible therapy in which sounds may be utilized to reduce anxiety in individual.
Introduction
Testosterone is one of the most important hormones in male that is produced 7 weeks after birth. The Level of this hormone rises during puberty, peaks at late teen, and then decreases later in life. Then after 30 years of age, testosterone level decrease each year. This hormone affects several systems such as reproduction, sexuality, muscle mass and bone density (Nieschlag et al. 2012). Testosterone induces the production of red blood cells and stimulates the growth of reproductive organ during puberty for sperm production. The effect of testosterone level on male behavior has not been fully established (Guo et al. 2013).
Testosterone and dihydrotestosterone are nuclear androgen receptors ligands (Heinlein and Chang 2002). The androgen receptor is a transcriptional factor which induces expression of genes that are involved in cell differentiation, metabolism and apoptosis (Hatzoglou et al. 2005). SF1 (steroidgenic factor 1) is a nuclear receptor and intracellular transcription factors that coded by nuclear receptor subfamily 5, group A, member (Taketo et al. 1995) and acute controller of reproduction (Jameson 2004). The increase level of SF-1 would lead to enhanced level of transcription of steroidogenic acute regulatory protein (StAR) and P450 aromatase genes in posterior hypothalamus and medial dorsal of brain (Goto-Kazeto et al. 2004). Upon binding of cytoplasmic Ca2+ to mitochondrial membrane steroidogenic acute regulatory protein, uptake of cholesterol into preplasmic space occurs which leads to production of testosterone (Stocco and Clark 1996). SF-1 in utero is a marker for growth and differentiation of neuroglial and sexual glands (Luo et al. 1994). During this period, SF1 is produced in steroid secreting glands and after birth, it is expressed in prosencephalon area of brain (Hammer et al. 1999; Ikeda et al. 1994). This protein is phosphorylated by protein kinase A and its activity is regulated with level of this modification (Hammer et al. 1999). The conversion of testosterone to estradiol by P450 aromatase in nerve cells is a key step in the differentiation of hippocampus, cortex, midbrain and amygdala. This enzymatic activity regulates physiology of reproduction in many mammals (Lephart 1996).
There are numerous factors which can change levels of testosterone that lead to memory impairment (Bimonte-Nelson et al. 2003). The production of testosterone is affected by various chemical agents. Alcohol reduces levels of expression of enzymes that are involved in synthesis of steroids, such as StAR, SF-1, P450scc, 3b HSD, 5a-reductase, P450aro and 3a HSD (Biggio and Purdy 2001). In contrast, level of testosterone is increased when a diet enriched with zinc ions (Zn2+) and branched-chain amino acids are consumed (Prasad et al. 1996). Intravenous injection of morphine in male rats induces expression of P450 aromatase gene. This enzyme is responsible for degradation of testosterone to estrogen which may cause behavioral changes (Aloisi et al. 2010). It has been demonstrated that sound waves act as a physical factor and may be capable of changing testosterone level in brain. Sound waves are divided into three groups based on their frequencies; Infrasound (10−4–20 Hz), hearing range (20–104 Hz) and ultrasound (2 × 104–1012 Hz). The range of frequency of ultrasound is widely used for treatment and detection of diseases has recently been experimented (Feril and Kondo 2004). It has been shown that excitation threshold frequency of 12, 48 and 90 Hz with intensities of 110 and 500 dB excites the temporal lobe. The mechanism of action of infrasound waves is unclear, but several studies demonstrate possible harmful effects of this waves (Alexander et al. 2010). The mice long-term exposure to 8 Hz frequency and 120 dB intensity significantly reduces testosterone concentration due to reduced level of SF-1 and StAR gene expression (Zhuang et al. 2007). As well as human study shown favorite music decrease testosterone level specially in male therefore such investigation demonstrated the sound a marked effects on hormone levels (Fukui 2001). The frequency of 261 Hz alters growth of human gingival fibroblasts cells. This wave stimulates and makes dramatic changes to membrane protein structure of tobacco cells by increasing the alpha-helix and a decreasing ß-turn content (dos Reis Lestard et al. 2013; Zhao et al. 2002). Effects of Sound waves at 1 kHz and 100 dB for 1 h within a distance of 20 cm could dramatically increase the cell wall fluidity, induce callus cells division (Liu et al. 2003). Sound waves stimulation could enhance activity of ATPase on plant plasma membrane and increase concentration of soluble proteins, sugars in callus (Wang et al. 2002). Therefore sound waves could increase the amounts of RNA transcript in planet cells. Exposure of sound wave with frequency of 8 Hz and intensity of 120 dB reduces stress and pain in cancer patients after tumor removal surgery (Good et al. 2010; Tan et al. 2010).
Testosterone could also act as an antioxidant and it reduces the effect of ROS. These species play a major role in various biological pathways, such as activation of various transcriptional factors, alter kinase and ion channel transportation activity (Sedeek et al. 2009). The reactive oxygen species (ROS); superoxide anion (O2·), hydrogen peroxide (H2O2), and the hydroxyl radical (–OH) are produced in oxidative metabolism and can cause destructive damage to biomolecules (Dröge 2002).
The significance of sound waves on key enzymes that regulate levels of testosterone in brain has not been investigated. The aim of our study was to examine physical stress of such as sound on induction behavioral changes in animal models. Testosterone is one of the most important hormones that have dramatic effects on behavior.
Methods
Primary cell cultures
All cells were cultured in 160 mL sterile flasks with DMEM medium (Gibco) which were supplemented with 10% Fetal Bovine Serum (FBS) and complemented with penicillin/streptomycin (50–100 µg/mL) at 37 °C and 5% CO2. The medium was generally changed every 2 days. The cell morphology changed to confocal after 11 days. At this time, medium was removed, cells were washed with DMEM and 1 ml of trypsin–EDTA (Gibco) (0.25%) solution was added and incubated for 5 min. Then cells were inactivated with 5 mL of complete media containing FBS for 3 min and spun at 300 g for 4 min and suspended in sterile phosphate buffer (pH 7.5) and enumerated by hemocytometer.
Cell viability
The numbers of viable cells were estimated with trypan blue staining.
Cells were washed twice with PBS-EDTA (10 mM in PBS) (pH 7.4) and re-suspended in 0.5% trypan blue (1 mL), and the number of viable cells was counted by hemacytometer. Cell viability was also assessed by utilizing dimethyl thiazolyl diphenyl tetrazolium (MTT) assay. 5 mg of MTT (Sigma Aldrich) was dissolved in 1 mL of phosphate buffered saline. An aliquot of 50 µL from supernatant of medium is removed in order to measure lactate dehydrogenase activity and then 5 µL of stock MTT was added to each well. After 4 h of storage at 37 °C, Reaction was stopped by addition of 100 µL DMSO or 0.04 N HCl in isopropanol solution. The purple precipitate from formazan chromophore was dissolved and measured by ELISA plate reader at a wavelength of 570 nm. The results were defined and expressed as a percentage of viability of control group. A p value of < 0.05 was considered statistically significant. The data were analyzed using one-way ANOVA.
Determination of ROS
ROS Production in cell culture was detected by utilizing dichlorodihydrofluorescein diacetate [DCDHF (Sigma Aldrich)] fluorescent probe. Cells cultures were loaded with 10 mM DCDHF in serum free medium (DEMEM) for 30 min at 37 °C. Then cells were washed and incubated in medium. Fluorescence spectroscopy was monitored by microplate at excitation of 485 nm and emission of 538 nm. A p value of < 0.05 was considered statistically significant. The data were analyzed using one-way ANOVA.
Lactate dehydrogenase enzyme (LDH) assay
This assay is conducted by the measurement of cytoplasmic lactate dehydrogenase enzyme (LDH), which is present in cell culture and upon damage to plasma membranes are by apoptosis or other factors, this enzyme is released to the medium. In summary, LDH was determined by diluting 10 µL of media to 2.60 mL with phosphate buffer (0.1 M, pH 7.4). After a short incubation at 37’C, the reaction was initiated in a cuvette by adding 100 µL of NADH (125 µM, final concentration) and 100 µL sodium pyruvate (300 pM, final concentration) to the diluted media. Then measurement was performed at 340 nm. The concentrations of enzyme in the media were determined by direct calculation based upon the decreases in absorbance. A p value of < 0.05 was considered statistically significant. The data were analyzed using one-way ANOVA.
Atomic absorption for Ca2+ determination
5% Solution of Lanthanum (La) was prepared by dissolving 58.6 g La2O3 in 250 mL of hydrochloric acid (1 mM) diluted with 1 L dd H2O. The stock solution of CaCO3 was prepared by dissolving 2.5 g CaCO3 in 50 mL of hydrochloric acid (0.1 M) diluted with 1 L dd H2O. The standard curve of this solution was made. The astrocyte cells were exposed to 1 h to sound waves with a frequency of 528 Hz and different intensity (80, 100 and 120 dB) in a distance from the ground to avoid possible vibration. The cells were centrifuged and their membrane was broken with ultrasonic bath. The solution was homogenized and then tested by atomic absorption for determination of Calcium ion concentration. A p value of < 0.05 was considered statistically significant. The data were analyzed using one-way ANOVA.
ELISA assay to testosterone determination
Brain tissues were homogenized with 90% methanol solution and centrifuged at 20,000g. Then supernatant was collected and evaporated by dry nitrogen. The total testosterone was evaluated with ELISA Kit (Abcam, ab108666). A 96-well plate was pre-coated with anti-Testosterone antibodies. The samples, along with the horse radish peroxidase (HRP) conjugated testosterone, were added to the wells. The testosterone in the sample competes with the added Testosterone-HRP for binding to antibody. After incubation, wells were washed to remove unbounded hormones and Tetra methyl benzoin (TMB) substrate was added for catalysis of HRP which consequently produces blue color. The reaction is terminated by addition of Stop Solution which stops the color development and changes the color from blue to yellow. The intensity of signal is inversely proportional to the amount of testosterone in the sample and is measured at 450 nm. A p value of < 0.05 was considered statistically significant. The data were analyzed using one-way ANOVA.
Animal
Twenty male rats (10 weeks old, weighting 160 ± 10 g) obtained from the University of Tehran Institute of Biochemistry and Biophysics (IBB) at an air-conditioned room (12/12 h light/dark cycle at a temperature of 23 ± 2 °C with relative humidity of 60 ± 5%). The animals were randomly divided into four groups (three groups for 80, 100 or 120 dB, respectively) and a control group. Experimental groups were exposed to 528 Hz sound wave at 80, 100 or 120 dB in the sonic chamber for 21 days 2 h a day (Zhuang et al. 2007). Whereas the control group was treated in the same manner, without any exposure to sound waves. All experiments were conducted along with the institutional ethical guidelines. All efforts were made to minimize the number of animals in group and any potential suffering of subjects.
Determination of gene expression level of SF-1, P450 aromatase and StAR
The qPCR reagents were purchased from GeneAll (South Korea). The primers were produced locally from Pishgam Tehran (Iran). Brain tissue were obtained from sacrificed rats at each time points, immediately immersed in liquid nitrogen and stored at − 80 °C until analyzed. Total RNA was isolated using TRIzol followed by chloroform extraction and isopropanol precipitation. 2 µg of total RNA was reverse-transcribed at 50 °C for 30 min in a reaction mixture which contains 0.5 mM dNTP, 400U of MMLV Reverse Transcriptase, 1 × RT buffer, 0.5 mM dithiotreitol and 0.5 g of oligo dT. Then RT enzyme was heat inactivated at 70 °C for 5 min and reaction was cooled to 4 °C. 4 µL were added to separate PCR reaction mixtures containing 0.1 mM dNTPs, 0.5U Taq DNA polymerase, 1 × PCR buffer with 3 mM MgCl2 and 25 pmol of the specific primers for the genes of steroidogenic factor 1 (SF-1), steroidogenic acute regulatory (StAR) protein and cytochrome P450 aromatase (P450aro). The primer pairs and expected products for individual genes are shown in Table 1. GAPDH primer pair was used as internal control. The PCR procedure was carried out using a GeneAmp 2400 PCR system under the following conditions: reverse transcription at 50 °C for 30 min, initial denaturation at 94 °C for 2 min, followed by 35 amplification cycles, each consisting of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s and extension at 72 °C for 30 s, with an additional extension step at the end of the procedure at 72 °C for 5 min. Dissociation curve analysis was performed after each assay to determine target specificity. Relative abundance was calculated as 2ΔCT, where ΔCT = Test CT − Control CT (Bustin 2002).Values were presented as mean SEM. The data were analyzed using one-way ANOVA. p values < 0.05 were considered statistically significant.
Elevated plus maze (EPM)
The EPM consists of two open and two closed arms. After 21 day of treatment with 528 Hz frequency at various intensities, Rats are tested in EPM and data related to time and number of entrance to each arm in 5 min was enumerated. A p value of < 0.05 was considered statistically significant. The data were analyzed using one-way ANOVA. The Open Arm time (OAT) and Open Arm Entrance (OAE) variation calculated by following formula (Eqs. 1 and 2):
Results
ROS in cell culture
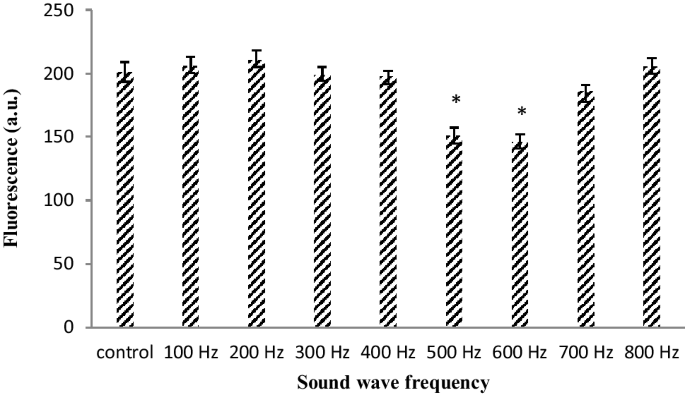
Astrocyte primary cell cultures were placed at distance of 20 cm under the sound waves with frequencies between 100 and 800 Hz and intensity of dB and then production of ROS was determined. The results shown that upon exposure to sound frequencies of 500 and 600 Hz, the ROS production was noticeably reduces (Fig. 1).
Different frequency of sound wave effects in ROS (The oxidation-sensitive fluorescent probe H2DCFDA was used to analyses the total intracellular content of ROS) production in astrocyte primary cell culture. 100 dB intensity reduced ROS production in 500 and 600 Hz frequency rather than other frequency. Data are shown as mean SEM of three separate experiments. Significant difference between treatment and control group at p ≤ 0.05 are presented as *
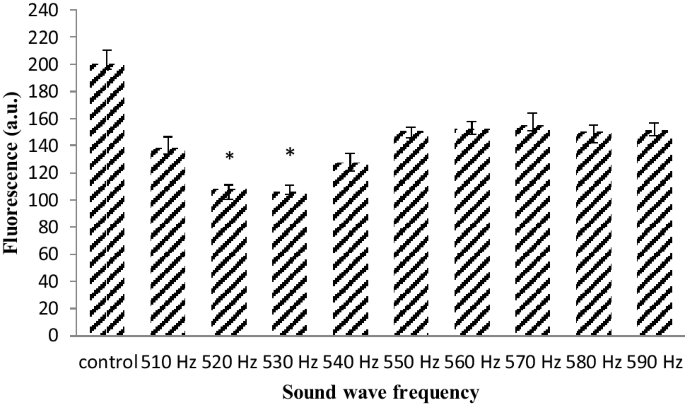
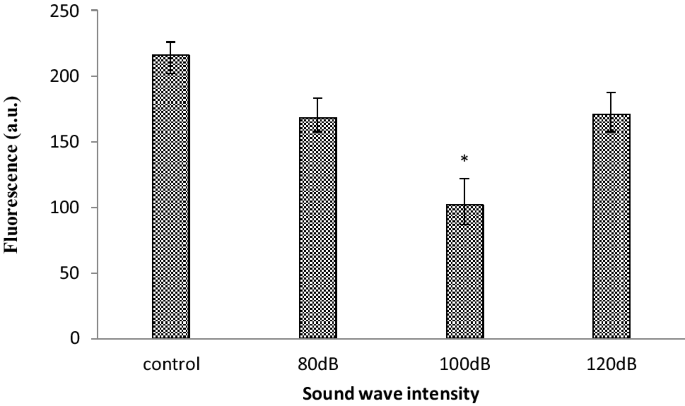
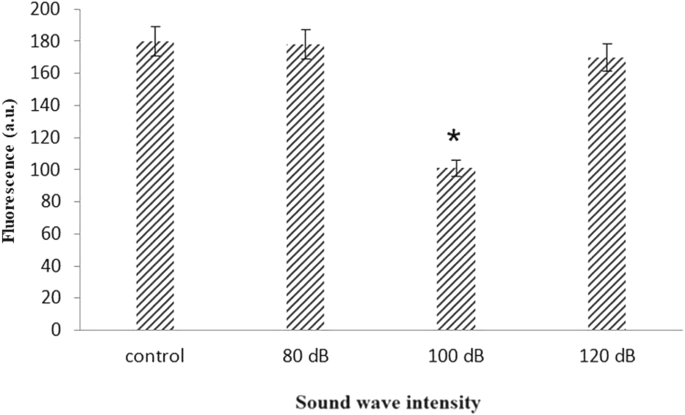
In order to confirm the reduction of ROS, the analysis was repeated with a finer range of frequencies between 510 and 590 Hz. The ROS production significantly reduces at 520 and 530 Hz frequency (Fig. 2). It has been documented that the frequency of 528 Hz sound wave reduces ROS (Babayi and Riazi 2017). Therefore this frequency was chosen for further analysis. The effect of this frequency was remarkable and the production of ROS was significantly reduced at 100 dB intensity. This reduction of ROS was lower than another two intensities of 80 and 120 dB (Fig. 3).
The different frequencies of sound wave effect in ROS (The oxidation-sensitive fluorescent probe H2DCFDA was used to analyze the total intracellular content of ROS) production in astrocyte primary cell culture. 100 dB intensity reduced ROS production in 520 and 530 Hz frequency rather than other frequency. Data are shown as mean SEM of three separate experiments. Significant difference between treatment and control group at p ≤ 0.05 are presented as *
The different intensities of 528 Hz sound wave significantly reduced ROS (The oxidation-sensitive fluorescent probe H2DCFDA was used to analyze the total intracellular content of ROS) production in astrocyte primary cell culture. 100 dB intensity reduced ROS production more than other intensity. Data are shown as mean SEM of three separate experiments. Significant difference between treatment and control group at p ≤ 0.05 are presented as *
MTT assay results
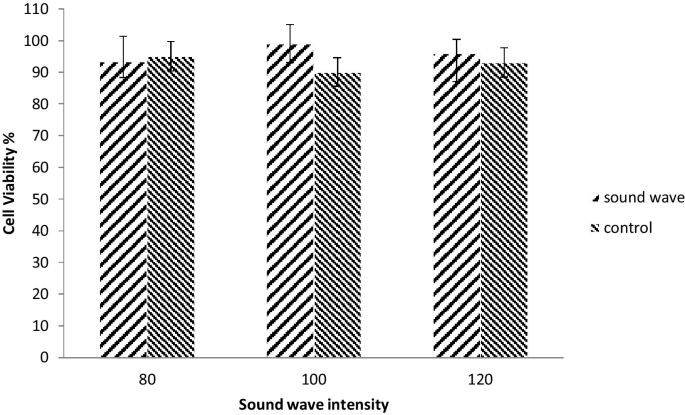
Astrocyte primary cell culture was exposed to intensities of 80, 100 and 120 dB of 528 Hz frequency of sound wave and then was tested for measuring cell viability by MTT assays (Fig. 4). After exposure to various intensities of sound wave, the viability of cells, according to MTT assay, did not change significantly,
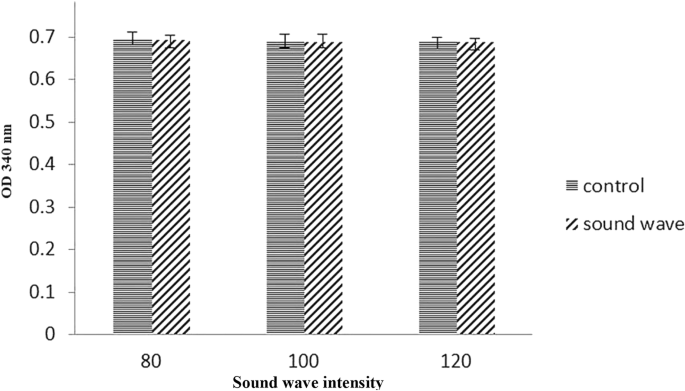
LDH assay results
Probably, The reduction of MTT results mainly occur due to presence of mitochondria through the action of succinate dehydrogenase. Therefore, it could provide a measurement of mitochondrial function (Slater et al. 1963). On the other hand, the reports indicate that reduction of MTT may also occur outside of mitochondria in some systems (Altman 1976). Therefore we used LDH assay for further analysis of cell viability. The loss of intracellular LDH and its release into the culture media is an indicator of irreversible cell death due to cell membrane damage. Similarly LDH assay indicated that, sound waves did not have a significant effect in lactate dehydrogenase activity in cell culture. Therefore, at distance of 20 cm, frequency of 528 Hz did not induce sufficient energy to disrupt cell membrane integrity (Fig. 5).
Evaluation of LDH activity in astrocyte primary cell showed that the activity of this enzyme after exposed to different intensities of 528 Hz frequency were not any significant changes. So the sound wave could not alter the LDH activity. Data are shown as mean SEM of three separate experiments. No significant difference between treatment and control group at p ≤ 0.05
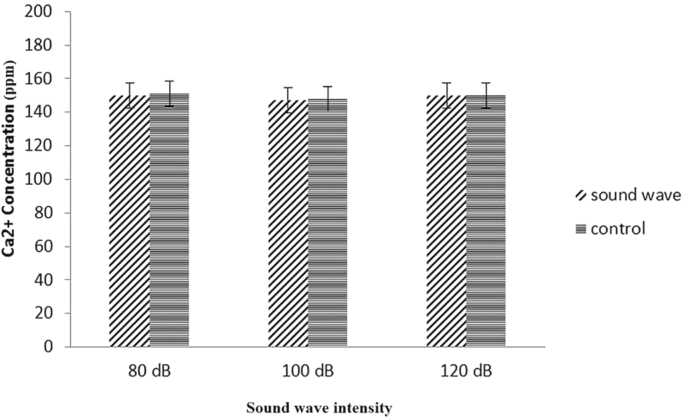
Calcium concentration in cell culture results
Astrocytes have many channels and receptors that are responsible for increasing the intracellular calcium concentration (Pasti et al. 1997). When membrane of astrocytes is depolarized the Ca2+ channels will be open. This Ca2+ influx through voltage-gated channels changes the action potential of astrocyte cell and it serves as the second messenger for initiation of many different cellular events. Intracellular calcium storage compartments could also attribute to increase in calcium concentration in cytoplasm (Stout et al. 2002). We hypothesized that under stimulation of sound waves, possible structural changes in calcium membrane channels and receptors could occur. These alterations can influence on uptake of calcium by these membrane proteins. After conjugation of cytoplasmic Ca2+ to mitochondrial membrane steroidogenic acute regulatory protein (StAR), uptake of cholesterol into preplasmic space will be increased. The enhanced uptake of calcium could increase the testosterone concentration. Detecting of intracellular calcium concentration done by using atomic absorption spectroscopy. The results did not demonstrate any significant difference between astrocyte primary cell culture control and cells that were treated with different intensity of 528 Hz frequency (Fig. 6).
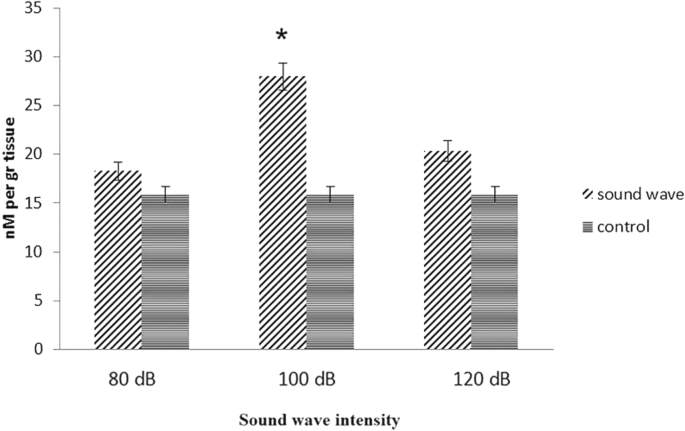
Testosterone concentration in brain
The produced testosterone in astrocyte cell culture was not detectable; due to limitation of assay detection (data not shown). Therefore, we used animal model to evaluate testosterone concentration by competitive sandwich ELISA assay with colorimetric method. Four groups, (consisting of five rats in each) were subjected to 528 Hz sound waves with intensities of 80, 100 and 120 dB and a control group was stimulated for 2 h per day for 21 days. The concentration of testosterone in this tissue increased significantly at 528 Hz sound wave frequency and 100 dB. The increment of testosterone was not significant at 120 and 80 dB (Fig. 7) as pronounced before.
Concentration of testosterone in brain tissue: testosterone concentration in exposed brain tissue to sound wave had significantly different with control groups after 21 days. Testosterone production increased significantly in frequency of 528 Hz with 100 dB. Data are shown as mean SEM of three separate experiments. Significant difference between treatment and control group at p ≤ 0.05 are presented as *
ROS in brain
Due to antioxidant properties of testosterone, it has been demonstrated that the reactive oxygen species (ROS) significantly reduced. Rats belong to experimental group (exposure to 528 Hz with intensities of 80, 100, 120 dB) were exposed to sound waves for 1 h and immediately the brain was extracted, homogenized and analyzed for detecting of produced ROS. The productions of ROS in brain at 528 Hz frequency with 100 dB is significantly reduced, however in other intensities ROS production was not any significant changes (Fig. 8).
The different intensity of 528 Hz sound wave significantly reduced ROS production in brain. 100 dB intensity reduced ROS production more than other intensity. Data are shown as mean SEM of three separate experiments. Significant difference between treatment and control group at p ≤ 0.05 are presented as *
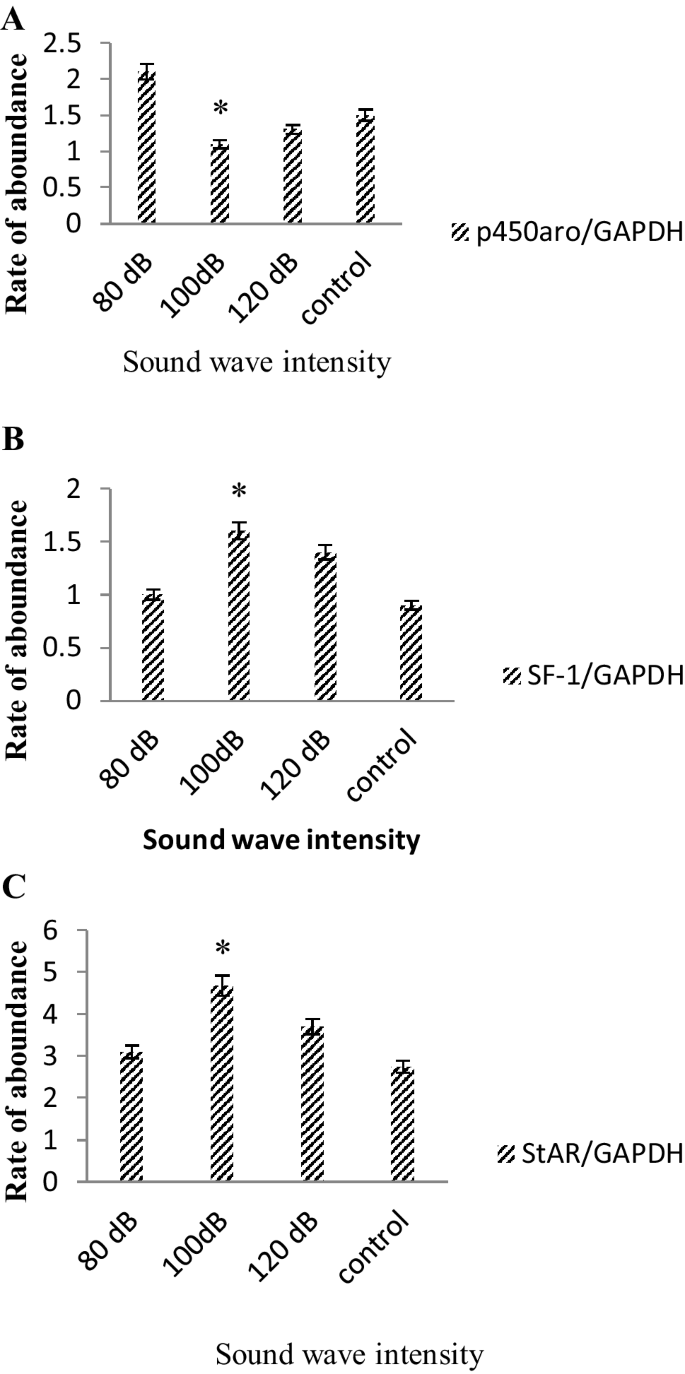
Gene expression results
The expression levels of StAR, SF-1, P450 aromatase and housekeeping gene GADPH were analyzed. The housekeeping gene GAPDH was utilized as internal control, the expression level of this gene must remain steady and constant. Any statistically meaningful variation from the control group indicates effect of sound in gene expression. The level of mRNA expression for StAR and SF-1 at 100 dB intensity demonstrated a significant increase compared with control group. At intensities of 80 and 120 dB the increment of StAR and SF-1 was less pronounced. On the other hand the level of mRNA expression of P450 aromatase gene was decreased profoundly only at experimental group treated with 100 dB and it did not change significantly for other intensities. The level of testosterone in brain tissue, which was markedly higher, is related to high expression level of StAR and SF-1 regulatory proteins at 100 dB. The mRNA level for P450 aromatase gene, which is involved with destruction of testosterone, remained steady at 80 and 120 dB intensities however it was decreased significantly at 100 dB (Fig. 9).
Behavioral results
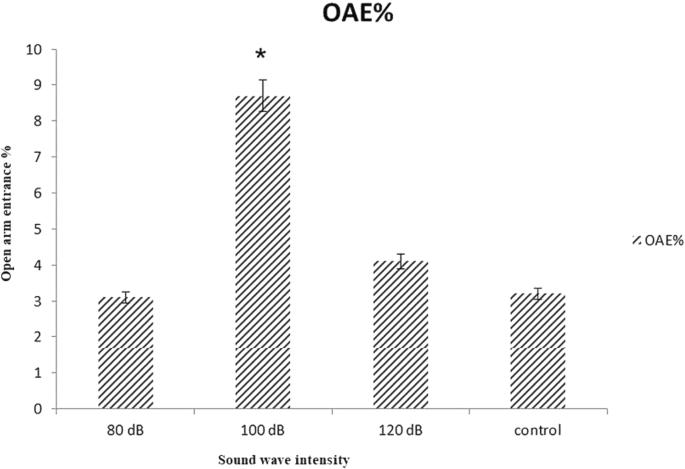
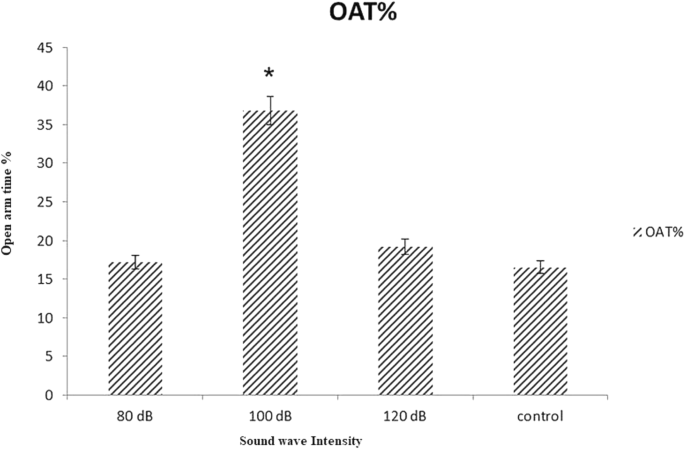
The results demonstrated that 100 dB intensity of 528 Hz sound wave increased percentage of time spent in open arms (OAT) and the number of open arm entries (OAE). There was not any significant variation in OAT or OAE in control, 80 and/or 120 dB treated groups. The apparent reason for reducing the anxiety is the increment of testosterone level in brain (Figs. 10, 11).
OAE% of rat in EPM after 21 days treated with 528 Hz frequency and 80, 100 and 120 dB intensity sound waves: There was significant difference between control and 100 dB intensity. Data are shown as mean SEM of five separate experiments. Significant difference between treatment and control group at p ≤ 0.05 are presented as *
OAT% of rat in EPM after 21 days treated with 528 Hz frequency and 80, 100 and 120 dB intensity sound waves: The significant difference between control and 100 dB intensity been observed. Data are shown as mean SEM of five separate experiments. Significant difference between treatment and control group at p ≤ 0.05 are presented as *
Discussion
It has been demonstrated that sound waves could have either a constructive or distractive effect on cellular viability. The viability of cells could be inversely affected by overproduction of ROS. The purpose of this study is to determine a range of frequencies which could have beneficial properties such as improved viability or reduction in production of ROS at cellular level. The final goal of this investigation is evaluation of behavioral alteration on rats upon daily exposure to a specific frequency and intensity. A wide range of frequencies from 100 to 800 Hz is selected for cellular exposure in order to estimate production of ROS. Since sound waves at low intensity, in 80 dB or less, have insufficient energy to induce significant effect, therefore 100 dB intensity was chosen for scanning and due to has sufficiency and low destructive effect was uniformly utilized.. Also in intensity over 100 dB the energy of sound wave may have destructive effects and could disrupt cell membrane integrity that leads to reduce cell viability. Previous research has shown that rats exposed to 90 dB and 8 Hz sound wave (infrasound) had declined sexual appetite due to decrease of testosterone production in testis. It was demonstrated that low level of testosterone in serum and testis in these rats was result of down regulation in StAR and P450scc gene expression that play a crucial role in hormone production (Zhuang et al. 2007). On the contrary, it appears that the sound wave at 528 Hz frequency which is sometimes referred to as miracle tone, could provide tranquility and a source for relieve of stress (Levi et al. 2015). It is interesting to note that this frequency has capability to alter physical interaction of each water molecules with another and might initiate formation of a cluster that could effectively enhance DNA repair (Horowitz 2000). Upon scanning a wide range of frequencies, it was demonstrated that sound waves at 528 Hz induces testosterone production in brain tissue. One of the chief effectors for relive of distress is production of testosterone in brain. The production of testosterone in brain is related to increase level of intracellular Calcium concentration. To elucidate a mechanism for change in concentration of testosterone in brain by 528 Hz sound wave, steroidogenic acute regulatory protein (StAR) and cytochrome P450 aromatase total mRNA expression were investigated. The acute and rate limiting step for regulating the synthesis of steroid hormones is delivery of cholesterol from intracellular storages vesicles to the inner mitochondrial membrane. The ionic interaction of Calcium with StAR protein permits transport of cholesterol through the mitochondrial membrane. The astrocytes cell culture exposed to 528 Hz frequency for 30 min did not demonstrate any significant changes in uptake of calcium. Therefore it seems that increased testosterone observed in this study entirely effects through altering in expression of key regulatory genes; StAR, p450 aromatase and SF-1. The tissue-specific transcription factor; steroidogenic factor 1 (SF-1), is a key regulator factor for expressing the steroid hydroxylase gene (Ingraham et al. 1994). Measuring the expression levels of steroidogenic factor 1 (SF-1) and StAR performed by quantitative RT-PCR showed that StAR and SF-1 mRNA expression levels in the exposed rats to 100 dB increased dramatically after 21 day. Meanwhile P450 aromatase mRNA expression levels decreased significantly in these rats. Therefore it appears that exposure to 528 Hz frequency with 100 dB intensity increases testosterone biosynthesis in brain by increasing of SF-1 level, improves its transportation of cholesterol through mitochondrial membrane and also retards its degradation by reducing level of P450 aromatase gene. Testosterone demonstrate antioxidant properties in rat nervous system (Ahlbom et al. 2001). The exposed homogenates of brain rats to 528 Hz frequency at 100 dB for 1 h exhibited reduction in ROS concentration compared to its experimental control counterpart. ROS production at physiological concentrations can trigger many signaling pathways (Kamogashira et al. 2015). Finally, rats which were exposed to a prolonged exposure of 528 Hz with 100 dB sound waves for 21 day were analyzed for behavioral alteration by elevated plus-maze. Our data confirm the research done by Aikey et al. who investigated the relation between testosterone level and anxiety (Aikey et al. 2002). Results of this study can be useful for next biological, molecular and psychological studies due to use sound wave with high efficacy and low destructive effect in body that naturally elevated testosterone levels.
References
-
Ahlbom E, Prins GS, Ceccatelli S (2001) Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res 892:255–262
-
Aikey JL, Nyby JG, Anmuth DM, James PJ (2002) Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm Behav 42:448–460
-
Alexander LD, Gilman DR, Brown DR, Brown JL, Houghton PE (2010) Exposure to low amounts of ultrasound energy does not improve soft tissue shoulder pathology: a systematic review. Phys Ther 90:14–25
-
Aloisi AM, Ceccarelli I, Fiorenzani P, Maddalena M, Rossi A, Tomei V, Sorda G, Danielli B, Rovini M, Cappelli A (2010) Aromatase and 5-alpha reductase gene expression: modulation by pain and morphine treatment in male rats. Mol Pain 6:1
-
Altman FP (1976) Tetrazolium salts and formazans. Prog Histochem Cytochem 9:1–56
-
Babayi T, Riazi G (2017) The effects of 528 Hz sound wave to reduce cell death in human astrocyte primary cell culture treated with ethanol. J Addict Res Ther 8:2
-
Biggio G, Purdy RH(2001) Neurosteroids and brain function. vol 46. Academic Press, New York
-
Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm A-CE (2003) Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp Neurol 181:301–312
-
Bustin S (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 29:23–39
-
dos Reis Lestard N, Valente RC, Lopes AG, Capella MA (2013) Direct effects of music in non-auditory cells in culture. Noise Health 15:307
-
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
-
Feril LB, Kondo T (2004) Biological effects of low intensity ultrasound: the mechanism involved, and its implications on therapy and on biosafety of ultrasound. J Radiat Res 45:479–489
-
Fukui H (2001) Music and testosterone. Ann N Y Acad Sci 930:448–451
-
Good M, Albert JM, Anderson GC, Wotman S, Cong X, Lane D, Ahn S (2010) Supplementing relaxation and music for pain after surgery. Nurs Res 59:259–269
-
Goto-Kazeto R, Kight KE, Zohar Y, Place AR, Trant JM (2004) Localization and expression of aromatase mRNA in adult zebrafish. Gen Comp Endocrinol 139:72–84
-
Guo W, Bachman E, Li M, Roy CN, Blusztajn J, Wong S, Chan SY, Serra C, Jasuja R, Travison TG (2013) Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell 12:280–291
-
Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, Weigel NL, Ingraham HA (1999) Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell 3:521–526
-
Hatzoglou A, Kampa M, Kogia C, Charalampopoulos I, Theodoropoulos PA, Anezinis P, Dambaki C, Papakonstanti EA, Stathopoulos EN, Stournaras C (2005) Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J Clin Endocrinol Metab 90:893–903
-
Heinlein CA, Chang C (2002) The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol 16:2181–2187
-
Horowitz LG (2000) Healing codes for the biological apocalypse. Tetrahedron Publishing Group, Sandpoint, pp 251–253
-
Ikeda Y, Shen W-H, Ingraham HA, Parker KL (1994) Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8:654–662
-
Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen W-H, Nachtigal MW, Abbud R, Nilson JH, Parker KL (1994) The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev 8:2302–2312
-
Jameson JL(2004) Of mice and men: the tale of steroidogenic factor-1. Oxford University Press, Oxford
-
Kamogashira T, Fujimoto C, Yamasoba T(2015) Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. BioMed Res Int 2015:617207
-
Lephart ED (1996) A review of brain aromatase cytochrome P450. Brain Res Rev 22:1–26
-
Levi R, Martinovsky G, Neuman G(2015) System and method for treating pets. United States patent US 9,107,389. 2015
-
Liu Y, Yoshikoshi A, Wang B, Sakanishi A (2003) Influence of ultrasonic stimulation on the growth and proliferation of Oryza sativa Nipponbare callus cells. Colloids Surf B 27:287–293
-
Luo X, Ikeda Y, Parker KL (1994) A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490
-
Nieschlag E, Behre HM, Nieschlag S(2012) Testosterone: action, deficiency, substitution. Cambridge University Press, Cambridge
-
Pasti L, Volterra A, Pozzan T, Carmignoto G (1997) Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci 17:7817–7830
-
Prasad AS, Mantzoros CS, Beck FW, Hess JW, Brewer GJ (1996) Zinc status and serum testosterone levels of healthy adults. Nutrition 12:344–348
-
Sedeek M, Hebert RL, Kennedy CR, Burns KD, Touyz RM (2009) Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curr Opin Nephrol Hypertens 18:122–127
-
Slater T, Sawyer B, Sträuli U (1963) Studies on succinate-tetrazolium reductase systems: III. Points of coupling of four different tetrazolium salts III. Points of coupling of four different tetrazolium salts. Biochim Biophys Acta 77:383–393
-
Stocco DM, Clark BJ (1996) Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmacol 51:197–205
-
Stout CE, Costantin JL, Naus CC, Charles AC (2002) Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277:10482–10488
-
Taketo M, Parker KL, Howard TA, Tsukiyama T, Wong M, Niwa O, Morton CC, Miron PM, Seldin MF (1995) Homologs of Drosophila Fushi-Tarazu factor 1 map to mouse chromosome 2 and human chromosome 9q33. Genomics 25:565–567
-
Tan X, Yowler CJ, Super DM, Fratianne RB (2010) The efficacy of music therapy protocols for decreasing pain, anxiety, and muscle tension levels during burn dressing changes: a prospective randomized crossover trial. J Burn Care Res 31:590–597
-
Wang B, Zhao H, Wang X, Duan C, Wang D, Sakanishi A (2002) Influence of sound stimulation on plasma membrane H+-ATPase activity. Colloids Surf B 25:183–188
-
Zhao H, Wu J, Xi B, Wang B (2002) Effects of sound-wave stimulation on the secondary structure of plasma membrane protein of tobacco cells. Colloids Surf B 25:29–32
-
Zhuang Z, Pei Z, Chen J (2007) Infrasound-induced changes on sexual behavior in male rats and some underlying mechanisms. Environ Toxicol Pharmacol 23:111–114
Acknowledgements
We thank prof. B. Bolouri for technical assistance and data analysis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest in this report.
Ethical approval
The authors assert that all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.