Case Study in Alleged Shaken Baby Syndrome
Louie Rodriguez suffered respiratory arrest at 5 months of age. He was hospitalized for 6 days and died. It was alleged that violent shaking of the head caused Louie’s injuries and death based on the finding of intracranial bleeding, brain edema, and bilateral retinal hemorrhage. Louie’s father was accused of killing Louie and arrested.
This investigation reveals that Louie’s respiratory arrest resulted from acute infections with Bordetella pertussis. His infections also caused weight loss, vitamin K deficiency, pneumonia, bleeding, metabolic acidosis, brain edema, and coma. A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that he had a white blood cell count of 79.5 x 103/µL and a lymphocyte count of 44.0 x 103/µL. His WBC and lymphocyte counts reduced by 90-95% following the treatment with antibiotic. Chest CT scan and X-ray exams showed that Louie had pulmonary hemorrhage, pulmonary edema, and lung disease. The standard diagnosed procedures that are used to diagnose B. pertussis infections in children were not utilized in this case.
Louie’s intracranial and retinal bleeding was caused by B. pertussis infection, septicemia, vitamin K deficiency, liver damage, pneumonia, and epinephrine. Increased intracranial pressure contributed to Louie’s retinal bleeding. Louie developed brain edema and edema in other locations as a result of anoxia and treatment with sodium bicarbonate. The allegations given that vigorous shaking of the head caused Louie’s injuries and death are not supported by medical facts. Louie’s illness and death were caused by acute infections with B. pertussis and septicemia.
Case Study in Alleged Shaken Baby Syndrome
ANALYSIS OF CAUSES THAT LED TO BABY LOUIE RODRIQUEZ’S PNEUMONIA, INTRACRANIAL AND RETINAL BLEEDING,
AND DEATH
by
Mohammed Ali Al-Bayati, PhD, DABT, DABVT
Introduction
Louie Rodriguez suffered respiratory arrest on December 13, 2006, and his father called 911. The EMS found Louie unresponsive with agonal (slowed irregular) respirations at 1613, and he was resuscitated. He had a heart rate of 200 beats/minute. There was no evidence of injury caused by trauma noted on Louie’s body. Louie was 5 months old and weighted 6800 g.
The EMS transported Louie to Providence Medical Center (PMC) in Kansas City and arrived at 1618. Louie was given 0.65 mL of epinephrine subcutaneously, IV fluid, atropine, and succinylcholine. He was intubated and transferred to Children Mercy’s Hospital on December 13th and arrived at 1642.
A Head CT scan exam performed on December 13th revealed that Louie had brain edema, subdural bleeding, and bilateral mastoid opacification. An eye exam performed at 1220 on December 14th revealed that Louie had retinal hemorrhage (new and old) in both eyes.
Louie was pronounced brain dead at 1306 on December 17th and remained on continuous mechanical ventilation until the life support was pulled on 12/19/2006. Dr. Erik Krag Mitchell performed the autopsy on Louie’s body on December 20, 2006 in Shawnee County Morgue in Topeka, Kansas.
Dr. Mitchell and Louie’s treating physicians alleged that violent shaking of the head (Shaken Baby syndrome) caused Louie’s injuries and death. Louie’s father was accused of killing Louie and arrested. Louie’s parents and their defense attorney requested this evaluation of the medical evidence in Louie’s case to find the likely causes that led to his illness and death.
The author is a toxicologist and pathologist with over 20 years experience in these fields, and has evaluated many cases of children who died suddenly from unexplained causes, including cases of children and adults who suffered from acute and/or chronic illnesses. These causes of illnesses and death required a differential diagnosis. The author has also served as an expert witness in many medical-legal cases involving children and adults. I have published over 50 articles in medical and scientific journals.
I evaluated Louie’s medical records, autopsy report, testimonies of expert witnesses and their reports, and the medical articles cited in this report using differential diagnosis. I also examined the H & E stained tissue sections of Louie’s organs prepared by the medical examiner. Approximately 250 hours were required to evaluate the medical evidence, perform an analysis, and write this report. My investigation in this case reveals the following:
1) Louie’s respiratory arrest occurred on December 13, 2006 resulted from acute infections with Bordetella pertussis. His infections also caused weight loss, vitamin K deficiency, pneumonia, bleeding, metabolic acidosis, brain edema, and coma. Below are biomarkers, clinical observations, and medical studies that show Louie was suffering from pertussis.
a) A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that he had a white blood cell count of 79.5 x 103/µL and a lymphocyte count of 44.0 x 103/µL. His lymphocyte count was 6 times the average normal level and he was suffering from severe lymphocytosis. Louie was treated with Ceftriaxone (antibiotic) IV infusion and his total white blood cells and lymphocyte counts reduced by 90% and 95% within 6 hours, respectively.
B. pertussis causes lymphocytosis and leukocytosis in children. For example, Lin et al. analyzed the clinical presentations of laboratory-confirmed B. pertussis infection of 46 cases (ages: 24 days to 37 years, with a mean of 4.3 years). Leukocytosis (white blood cells > or = 15 x 103/µL) and lymphocytosis (lymphocytes > or = 10 x 103/µL) were observed in 17 and 16 of the individuals, respectively. Fourteen individuals (30.4%) developed complications, among which pneumonia was the most common (92.3%) among infants < or = 1 year of age (Section 6).
b) Chest CT scan and X-ray exams performed within 2 hours following Louie’s admission to the hospital showed that Louie had pulmonary hemorrhage, pulmonary edema, and lung disease. B. pertussis causes pneumonia and pulmonary hemorrhage in children. Fauci et al. stated that the major respiratory complication of pertussis is pneumonia and it is more common in infants (incidence, 21 percent). B. pertussis produces a variety of toxins that impair local defenses, inhibit phagocytosis, and cause local tissue damage and bleeding (Section 6).
c) Louie developed apnea, cynosis, tachycardia, sunconjunctival hemorrhage, respiratory failure, and seizure on December 13th and these symptoms have been reported in infants with pertussis. Fauci et al. stated that episodes of cynosis and apnea are common among infants and small children suffering from pertussis (prevalence, 20 to 50 percent). In addition, B. pertussis infection in infants causes sunconjunctival hemorrhage and upper torso petechiae due to increased intrathoracic pressure (Section 6).
d) Infection with B. pertussis causes severe hypoxia and metabolic acidosis. Louie’s blood pH was 6.85 and suffered from a severe metabolic acidosis. McCarthy and Carlile stated that B. pertussis causes hyperleukocytosis in infant and hyperleukocytosis with pulmonary leukostasis can result in significant hypoxemia. Metabolic acidosis causes brain edema and coma. Louie was treated with sodium bicarbonate IV and his blood pH rose to 7.55 within 4 days (Section 6).
e) A head CT scan taken at 2 hours following Louie’s admission to the hospital showed that he had encephalopathy. Acute infection with B. pertussis has caused neurologic complications in infants that include encephalopathy (0.7%) and seizures (2 percent). The potential mechanisms of pertussis-associated encephalopathy were postulated to include hypoxia and/or hypoglycemia due to pertussis toxin, hemorrhages secondary to increased venous pressure, direct neurotoxic effects, and coinfection by neurotoxic virus (Section 6).
f) Malnutrition and weight loss are among the complications of infections with B. pertussis in infants. Louie’s weight on December 11th and December 13th was 7000 and 6800 g, respectively and had lost 200 g in 2 days. Louie’s weight gain rate between November 28th and December 11th was 73.1 g/day.
2) Louie’s treating physicians and the medical examiner did not utilize the standard diagnosed procedures that are used to diagnose B. pertussis infections in children.
The standard diagnostic test used is the isolation of B. pertussis from nasopharyngeal swab culture. A calcium alginate swab is usually inserted into the nares of the child and then placed in to transport medium such as Regan-Lowe charcoal medium or plated directly onto fresh Bordet-Gengou agar or another suitable agar. Growth typically requires 3 to 5 days of incubation at 36 oC. Suspicious colonies usually identified by direct fluorescent antibody staining or agglutination.
3) Louie’s intracranial and retinal bleeding was caused by B. pertussis infection and septicemia, vitamin K deficiency, liver damage, pneumonia, and his treatment with high doses of epinephrine as indicated by the following clinical observations.
a) Louie had elevated level of alkaline phosphatase (AlkP) and glutamic oxaloacetic transaminase (GOT) in serum that indicates liver damage. A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that his ALK and SGOT levels in serum were equal to 161% and 323% of the upper normal level, respectively. Liver plays a central role in the clotting process (Section 7).
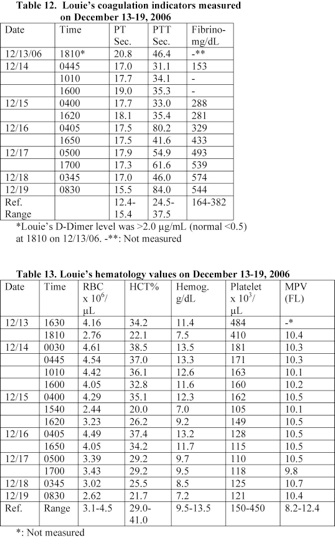
b) A blood analysis performed at 2 hours following Louie’s admission to the hospital revealed that he had elevated prothrombin time (PT) and partial thromboplastin time (PTT). He had a PT of 20.8 seconds (normal: 12.4-15.4) and a PTT of 46.4 seconds (normal: 24.5-37.5). In addition, his PTT reached a very high level of 80.2 seconds on December 16th. PT measures clotting factors II, V, VII, X and fibrinogen and these factors are synthesized in the liver.
c) Intracranial bleeding and bleeding in other locations have been reported in children suffered from vitamin K deficiency. For example, 1) Nishio et al. examined 84 cases of intracranial hemorrhage in children due to vitamin K deficiency from literatures. Hemorrhage sites were identified by CT scan in these children. Subarachnoidal hemorrhage was in 72 cases (85.7%), subdural hemorrhage was in 41 cases (48.8%), intracerebral hematomas was in 36 cases (42.9%) and intraventricular hemorrhage was in 9 cases (10.7%) (Section 7).
d) Septicemia is frequently accompanied by changes in the plasmatic as well as cellular coagulation systems and by microclot formation. Louie suffered from coagulation problem and DIC. His level of D-Dimer was more than 4 times the normal level and his platelet count reduced by 63% within 6 hours (Sections 4, 7).
e) Louie was given 0.65 mL of epinephrine by subcutaneous route. Intracranial bleeding has been reported in some children and adults treated with high therapeutic doses of epinephrine (Section 7).
f) Louie developed bleeding following his admission to the hospital on December 13th. A blood analysis performed at 17 minutes following admission to the hospital revealed that Louie’s hematological values were within the normal range. However, his blood analysis performed at about 2 hours following admission indicates that he developed anemia. His RBC and hemoglobin level reduced by 34%.
Furthermore, Louie received RBC transfusions prior to 0030 on December 14th and his RBC, hematocrit value, and hemoglobin level increased and reached a normal level. However, his blood analysis performed at 1540 on December 15th showed that he developed anemia due to bleeding. A significant amount of blood was detected in his urine (Section 4).
g) Louie developed subarachnoid bleeding following his admission to the hospital on December 13th. The subarachnoid bleeding observed at autopsy was not shown on the head CT scan performed at 2 hours following Louie’s admission to the hospital.
4) Louie developed brain edema and edema in other locations following his admission to the hospital. The ME reported that Louie’s calvarial sutures were widely separated up to a maximum of approximately 1 inch and bulge. A head CT scan exam performed following Louie’s admission on December 13th did not show that he had separation of the cranial sutures. Louie’s head circumference (HC) on December 14th and at autopsy was 42 and 45 cm, respectively.
In addition, Louie’s weight on December 13th and at autopsy was 6800 and 8190 g, respectively. His weight increased by 20.44% during his 7-day hospitalization.
Louie’s length on December 14th and at autopsy was 59 and 65 cm, respectively. His length increased by 6 cm during 6 days of hospitalization. These data indicate that Louie developed severe body edema following his admission to hospital on December 13th.
Anoxia, septicemia, metabolic problems, treatment with high doses of sodium bicarbonate, and kidney problems caused Louie’s severe edema.
5) The allegations given that vigorous shaking of the head caused Louie’s injuries and death are not supported by medical facts. Louie’s illness and death were caused by acute infections with B. pertussis and septicemia.
2. Louie’s premature birth and his 3-week hospitalization following birth
Louie was born on July 11, 2006 at St. Joseph’s Hospital in Tucson, Arizona. He was born at about 33 weeks gestation by C-section for breech presentation. His Apgar scores at 1 and 5 minutes were 3 and 9, respectively. He required positive pressure ventilation. His weight was 2044 g. His length and head circumference were 41 and 30.5 cm, respectively [1].
The pregnancy was complicated by vaginal bleeding in the first trimester and premature rupture of membranes. Louie’s mother received steroids and antibiotics prior to delivery. Louie’s mother was 27 years of age and has a history of asthma, irregular heart beat, kidney stones, and resolved kidney failure.
Shortly after birth, Louie was transferred to the Neonatal Intensive Care unit (NICU) for increasing respiratory distress and stayed until August 3, 2006. His respiratory distress was secondary to transient tachypnea of the newborn/retained fetal lung fluid. Louie required oxygen administration by oxyhood but remained on room air through the majority of his hospitalization.
Louie also suffered from bradycardia of prematurity and hypotension. His hypotension was resolved on day 1 following birth. Louie had significant leukocytosis and treated with ampicillin and gentamicin for a total of 10 days (Table 1).
Louie was also diagnosed with hypoglycemia and hypocalcemia following birth and treated with glucose and calcium gluconate IV (Tables 2, 3). He developed jaundice and treated with phototherapy for 4 days. His maximum serum total bilirubin level was 9.5 mg/dL. Louie and his mother have O positive blood type and his direct Coombs test revealed negative result.
Louie’s hematology values were within the normal ranges during his 24 days following birth. However, his hematocrit reduced from 56% to 41% during the 20 days following birth (Table 4). He was treated with ferrous sulfate and multivitamins. Louie’s screening head ultrasound exam was normal and he passed his newborn hearing screen.
Louie was discharged from the hospital on August 3rd. His weight and head circumference were 2405 g and 42.5 cm, respectively. His weight gain rate and head circumference growth rate during the 23 days following birth were 15.7 g/day and 2.61 cm/month, respectively.
3. Louie’s health condition between August 4th and December 13, 2006
Louie was discharged from the hospital on August 3, 2006. The review of his medical records shows that he was taken to the hospital 3 times between September 22nd and December 11, 2006 due to inconsolable crying, developing skin rash and eczema, allergy, and wheezing. Louie was treated with Albutrol, corticosteroid, and Bendryl on December 11th. He suffered from respiratory arrest on December 13th and hospitalized [2].
3.1 Louie’s eye problem on September 22, 2006 and treatment given
Louie was fussy and cried on September 22, 2006 and his parents took him to the ER at the Providence Medical Center (PMC) in Kansas City. Physical exam revealed that Louie had corneal abrasion at the right eye and treated with erythromycin ointment. Louie’s weight was 4830 g and he had gained weight at the rate of 48.5 g/day since he was released from the hospital on August 3, 2006 (Table 5).
3.2 Vaccines given to Louie
Louie received the following vaccines on October 2, 2006: Diphtheria, Tetanus, and pertussis (DTaP); Haemophilus influenzae type b (Hib); Pneunococcal conjugate (PCV), and hepatitis B. He also received hepatitis B vaccine on August 29, 2006.
3.3 Louie’s skin rash and eczema on November 28, 2006 and treatment given
Louie developed skin rash and eczema on November 28, 2006 and was taken to the ER at PMC. He was noted to have eczema around his eyes. He also had lesions on his arms and legs. He had a temperature of 100.3 oF, a pulse of 140/minute, and a respiratory rate of 44/minute [2, 3]. He was treated with topical steroids [4].
Louie’s weight was 6050 and he had gained weight at the rate of 18.20 g/day since September 22, 2006. His length and head circumference (HC) were 55.9 and 41 cm, respectively. His length and HC increased at the rate of 3.19 and 2.25 cm/month, respectively (Table 5).
3.4 Louie’s allergy and wheezing on December 11, 2006 and treatment given
Louie developed facial swelling and breathing problems (wheezing) on December 11th. His mother took him to PMC at 1526. Examination revealed that Louie’s face and eyes were swollen and had facial edema. He also had mild wheezing. He had a temperature of 98.2 oF, a pulse of 144/minute, and a respiratory rate of 48/minute.
Louie’s chest X-ray exam was normal. He was diagnosed as having an acute allergic reaction. Louie was allergic to pineapple and he broke out in a rash after his pacifier was dipped in the pineapple juice. He received Benadryl, steroids, and breathing treatment (Albuterol/Atrovent).
Louie’s weight was 7.0 kg on December 11th and he had gained weight at the rate of 73.1 g/day since November 28th, which is equal to 401% of his weight gain rate between September 22nd and November 28th. These data indicate that his body was retaining fluid.
3.5 Louie’s respiratory arrest on December 13th and the EMS response
Louie took less than half of the usual amount of formula on December 13, 2006. He normally takes a full 8-ounce bottle of formula milk with each meal but he took 2 to 3 ounces of formula at 0830 and 1230. He was also fussy.
Louie’s mother left home at about 1500 while Louie was sleeping. Louie woke up from his nap crying and his father changed his diaper. The father left the baby on the bed and went into the kitchen. He came back a few minutes later and found Louie was not breathing, limp, and turned blue.
The father called 911 and he was given instruction to blow air in Louie’s mouth. Blood came out from Louie’s nose. Louie’s mother returned home prior to the arrival of EMS and began mouth-to-mouth breathing. The baby began to take agonal breath [2, 4].
The EMS arrived at the seen at 1558 and found Louie unresponsive with agonal respirations. They used a portable suction unit with catheter and sucked out clear secretions. The tip of the suction catheter had bright red blood on it. They initiated bag-valve mask ventilation and placed Louie on oxygen. He had a heart rate of 200 beats/minute.
Examination revealed that Louie had red and blotchy looking rash on his chest area and toward the back. His cheeks were red and had dry skin. His pupils were dilated.
They gave him lactated Ringer solution via intraossesous infusion. They transferred Louie to PMC and arrived at 1613. The baby was noted to have posturing movements during transportation [2, 4].
Louie’s weight on December 13th was 6800 g and had lost 200 g since December 11th (-100 g/day). However, his length increased at the rate of 6.20 cm/month, which is equal 200% of his length increase rate for the period between July 11th and September 22nd (Table 5).
4. Louie’s hospitalization on December 13-19, 2006, clinical tests, health problems, and treatments given
Louie arrived at the Providence Medical Center’s ER at 1613 on December 13, 2006. He was bagged by bag-valve mask and had agonal respirations. His heart rate was 180 beats/minute and had tachycardia. His temperature was 35.2 oC. Louie was given 0.65 mL of epinephrine (1/1000) subcutaneously at 1618 [2-4].
Examination revealed that Louie had subconjunctival hemorrhage and his abdomen was soft with absent bowel sounds and no organomegally. His extremities were cool peripherally. There was no evidence of injury caused by trauma noted on Louie’s body.
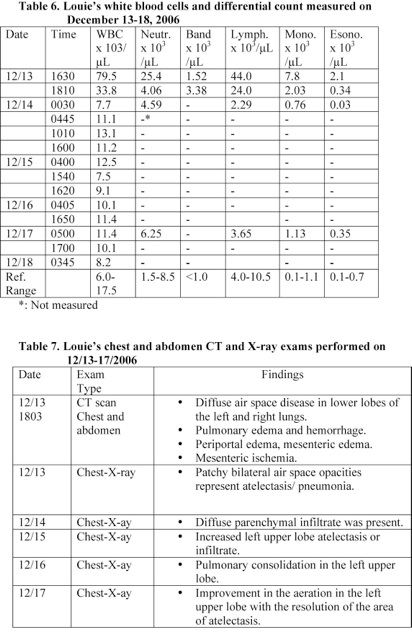
A blood analysis performed at 1630 revealed that Louie had a white blood cells count of 79.5 x 103/µL and a lymphocyte count of 44.0 x 103/µL. Louie’s lymphocyte count was 6 times the average normal level and was suffering from severe lymphocytosis (Table 6).
Chest CT scan and X-ray exams performed within 2 hours following Louie’s admission to the hospital revealed that he had pulmonary hemorrhage, pulmonary edema, and lung disease (Table 7). He was given Ceftriaxone IV infusion and his total white blood cells and lymphocyte counts reduced by 90% and 95% within 6 hours, respectively (Table 6).
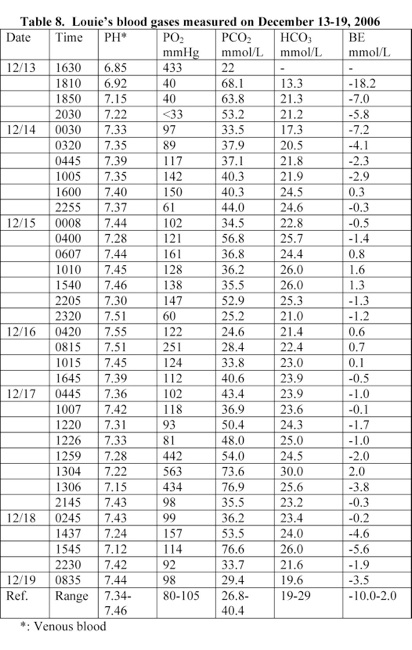
Louie’s blood pH was 6.85 and was suffering from a severe metabolic acidosis. He was treated with sodium bicarbonate IV and his blood pH rose to 7.55 within 4 days (Table 8). Louie had an elevated serum potassium level of 6.9 mEq/L, glucose level of 336 mg/dL, anionic gap of 27 mmol/L, alkaline phosphatase level of 403 U/L, and
glutamic oxaloacetic transaminase level of 110 U/L (Tables 9-11)
Louie was given atropin and succinylcholine and intubated at 1637. He was transferred to Children Mercy’s Hospital (CMH) on December 13th and arrived at 1642. Examination revealed that his head was normocephalic and atramatic. The anterior fontanel was bulging and tense. He had a heart rate of 178/minute and a blood pressure of 106/79 mm Hg [5].
Louie started to have seizure like activity and was given fosphenytion and Ativan. He was also given dopamine/milrinone for cardiac support and Benadryl for allergy. In addition, he was given CaCl2, isotonic fluids, 5% albumin, and steroids (IV). His weight was 6.8 kg.
A blood and urine analyses performed at 1810-30 on December 13th revealed that Louie was suffering from coagulation problems and had hematouria. He developed anemia and hypoprotenemia. Louie’s prothrombin time (PT) and partial thromboplastin time (PTT) were elevated. His PT and PTT were 20.8 and 46.4 seconds, respectively (Table 12). He was given fresh-frozen plasma (FFP), and vitamin K, IV.
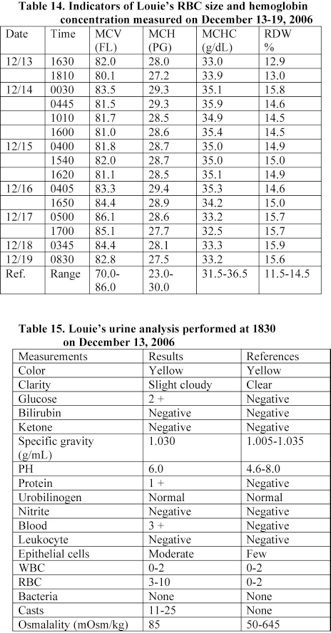
Louie’s red blood cell count, hematocrit value, and hemoglobin level were 2.76 x 106/µL, 22.1%, and 7.5 g/L, respectively. His RBC and hemoglobin level reduced by 34% of the values measured following his admission to the hospital at 1630 (Table 13). In addition, Louie’s urine analyzed at 1830 revealed that he had a significant amount of blood and a trace amount of protein in the urine (Table 15). He received red blood cell transfusion.
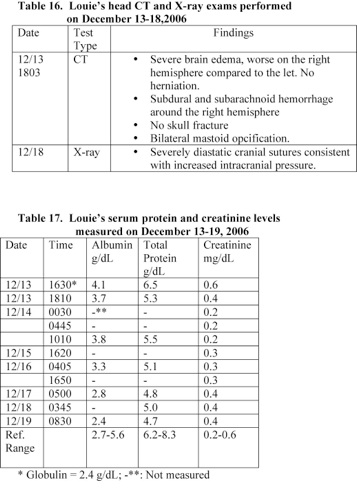
A Head CT scan exam performed on December 13th revealed that Louie had severe brain edema (worse in the right hemisphere than the left), subdural bleeding, and bilateral mastoid opacification (Table 16). Louie was treated with zantac, morphine, versed, dopamine, and phenobarbital.
An eye exam performed at 1220 on December 14th revealed that Louie’s pupils were dilated and nonreactive bilaterally. Retinal hemorrhage was noted on fundoscopic exam bilaterally.
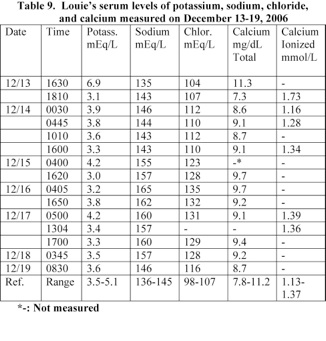
Louie developed hypernatremia and hyperchloremia on December 15th. His serum sodium and chloride levels were 158 and 128 mEq/L, respectively (Table 9). He showed signs of diabetes insipidus on December 15th. He produced a large volume of urine and was treated with DDAVP.
Louie’s platelet count reached a low level of 105 x 103/µL on December 15th. His platelet count on December 13th was 484 x 103/µL and he lost 78% of his platelet within 2 days due to bleeding and Disseminated Intravascular Coagulation (DIC) (Table 13). An old blood was suctioned from his lungs. Louie’s weight and head circumference on December 15th were 6.8 kg and 42.5 cm, respectively.
The treating physicians alleged that Louie’s injuries were resulted from vigorous shaking of the head (Shaken Baby Syndrome) and his father was accused of causing the injuries and arrested on December 14, 2006. Louie was pronounced brain dead at 1306 on December 17th. He had two electroencephalogram (EEG) exams that confirmed this diagnosis.
Louie remained on continuous mechanical ventilation until the life support was pulled off at 1436 on 12/19/2006. An autopsy was performed on December 20th inWynadotte County, KS. The clinical data collected during Louie’s 7 days hospitalizations are presented in Section 4.1-12.
4.1 Evidence of bacterial infections and pneumonia
A blood analysis performed within 17 minutes following Louie’s admission to the hospital revealed that he was suffering from severe lymphocytosis. His lymphocyte count was 6 times the average normal value. His neutrophil, monocyte, and esoinophil counts were also elevated (Table 6). Louie was treated with antibiotic and his total white blood cells and lymphocyte counts reduced by 90% and 95%, respectively within 6 hours.
Chest CT scan and X-ray exams performed within 2 hours following admission revealed that Louie had pulmonary hemorrhage, pulmonary edema, and lung disease. His CT exam of the abdomen showed periportal edema and signs of mesenteric ischemia. Louie’s chest-X ray exams taken on December 14, 15, and 16th also showed that he had diffuse lung disease (Table 7). These data indicate that Louie was suffering from acute Bordetella pertussis infection and septicemia [6-13].
4.2 Metabolic acidosis
A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that he was suffering from a severe acidosis. His blood pH was 6.85. He was treated with sodium bicarbonate IV and his blood pH rose to 7.55 within 4 days (Table 8).
4.3 Hyperkalemia, hypernatremia, and hyperchloremia
A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that his serum potassium level was elevated and retained to a normal level following his treatment with sodium bicarbonate. Louie developed hypernatremia and hyperchloremia at 36 hours following admission (Table 9).
Louie’s phosphorous and magnesium levels were 6.7 mg/dL (normal: 4.2-7.0) and 2.3 mg/dL (1.6-2.3) at 1810 on December 13th. His serum osmolality was 315 Osm/kg (normal: 275-296 m Osm/kg) at 0607 on December 15th.
4.4 Hyperglycemia and elevation of anion gap
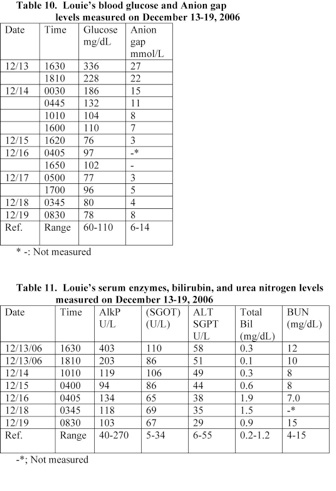
A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that he was suffering from hyperglycemia. He also had an elevated anion gap of 27 mmol/L. His glucose and anion gap level reduced to a normal level within 18 hours following admission (Table 10). Louie had a normal serum lipase level of 45 U/L (normal range: 23-300 U/L) at about 2 hours following admission.
4.5 Indication of liver damage
A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that he had elevated serum levels of alkaline phosphatase (AlkP) and glutamic oxaloacetic transaminase (GOT). The level of the ALKP reduced to a normal level within 90 minutes following Louie’s treatment with antibiotic (Table 11).
4.6 Indicators of blood coagulation
A blood analysis performed at 2 hours following Louie’s admission to the hospital revealed that he had elevated levels of prothrombin time (PT) and partial thromboplastin time (PTT). His D-Dimer level was also elevated (Table 12). These data indicate that he was suffering from coagulation problem and Disseminated Intravascular Coagulation (DIC). He received vitamin K and frozen fresh plasma (FFP), IV.
4.7 Development of anemia and thrombocytopenia
A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that his hematological values were within the normal range (Tables 13, 14). However, his blood analysis performed at about 2 hours following admission indicates that he developed anemia. His RBC and hemoglobin level reduced by 34% (Table 13).
Louie suffered from coagulation problem and a significant amount of blood was detected in his urine (Table 15). In addition, he developed DIC. His level of D-Dimer was more than 4 times the normal level and his platelet count reduced by 63% within 6 hours (See: Tables 12, 13).
Louie received red blood cell transfusions prior to 0030 on December 14th and his red blood cell count, hematocrit value, and hemoglobin level increased and reached a normal level. However, his blood analysis performed at 1540 on December 15th shows that he developed anemia due to bleeding (Table 13).
Louie’s blood analysis performed at 1818 on December 15th showed that his blood is O+ and antibody screen negative. Louie’s anemia caused by bleeding occurred following his admission to the hospital.
4.8 Hematouria, glucoseuria, and proteinuria, ketoacidosis
A urine analysis performed at 2.25 hours following Louie’s admission to hospital showed that he had a high level of blood in his urine. A moderate amount of glucose and trace amount of protein also present in his urine (Table 15).
Louie’s urine analysis performed on December 13th did not show the presence of ketone bodies. However, his urine analysis performed at 1700 on December 14th revealed increased level of acetoacetic and 3-hydroxybutic acids with secondary elevation of dicarboxylic acids, which indicates that Louie was suffering from ketosis
4.9 Head CT scan and X ray exams
Louie’s head CT exam performed on December 13th showed extensive edema and small subdural hemorrhages. No signs of hernination or skull fractures were seen at this time. However, his head X-ray exam performed within 6 days following admission revealed widening of the cranial sutures (Table 16).
4.10 Eye exam
An eye exam conducted at 1220 on December 14th revealed that Louie had focal subconjunctival hemorrhage and diffuse retinal hemorrhaging (old and fresh) in both eyes. The optic nerves were without edema.
4.11 Serum protein and creatinine levels
A blood analysis performed at 1630 on December 13th revealed that Louie had normal serum albumin and creatinine levels. However, his serum total protein reduced to a level below the normal range (Table 17).
4.12Screen for metabolic disorders
Louie’s blood was examined for metabolic disorders and revealed negative result for the following: Acylcarnitine profile, CAH 17-OHP, congenital hypothyroidism-TSH, and galactose-(Gal and Gal-1-p).
5. Autopsy and histopathology findings in Louie’s case
Dr. Erik Krag Mitchell performed the autopsy on Louie’s body at 1440 on December 20, 2006 in Shawnee County Morgue in Topeka, Kansas. External examination did not reveal any evidence of injury caused by trauma. An X-ray exam of Louie’s body in the morgue did not show that Louie had broken bone or bone anomalies [14].
Dr. Mitchell examined Louie’s organs grossly and microscopically and alleged that Louie’s death was caused by violent shaking of the head (Shaken Baby syndrome). He based his opinion on the finding of diffuse severe brain edema, subdural and subarachnoid bleeding, and retinal bleeding [14]. His main findings are described in Section 5.1-6.
My review of the clinical data, medical studies, autopsy and histopathology findings, and examination of the H & E stained tissue sections (received from the medical examiner’s office) indicate that Louie suffered from severe acute pulmonary infections and septicemia caused by Bordetella pertussis. His infections caused severe metabolic acidosis, brain edema, and respiratory arrest (Section 6).
Anoxia, sodium bicarbonate, and irritation from bleeding caused louie’s severe brain edema. Louie’s intracranial and retinal bleeding resulted from infections, liver problems, and epinephrine given in the hospital. In addition, increased intracranial pressure contributed to Louie’s retinal bleeding (Section 7).
5.1 Examination of Louie’s body and measurements
The medical examiner (ME) stated that Louie’s body was fairly obviously edematous with flat pressure reduction of edema on the right side of the back of the head. His eyelids were swollen and edematous to the point that his conjunctivae cannot be adequately examined. There was no indication of remote injury or acute injury of Louie’s chest, abdomen, lower extremities, or upper extremities. An X-ray examination of the body demonstrated no skeletal fracture.
Louie’s body’s weight and length were 8190 g and 65 cm, respectively. He had a head circumference (HC) of 45 cm. Louie’s weight following admission on December 13 was 6800. Louie’s weight increased by 20.44% during his 7-day hospitalization.
In addition, Louie’s length and HC on December 14th were 59 and 42 cm, respectively. His length and HC increased by 6 and 3 cm, respectively during 6 days of hospitalization due to fluid retention. These data indicate that Louie developed severe body edema following his admission to hospital on December 13th.
5.2 Examination of Louie’s skull and the brain
The ME reported that Louie’s scalp had red discoloration following suture lines. His calvarial sutures were widely separated up to a maximum of approximately 1 inch and bulge. Louie’s brain was very soft and was not weighted.
There was diffuse engorgement of the meninges with the films of subarachnoid and subdural blood in the anterior fossa and over the vertex. There was also blood in the posterior fossa. The brain was not fixed and no tissue was taken for histological evaluation.
A head CT scan exam performed following Louie’s admission to the hospital on December 13th did not show that Louie have separation of the cranial sutures (Table 16). The separation of cranial sutures occurred in the hospital due to increased intracranial pressure caused by edema resulted from anoxia and the treatment with high doses of sodium bicarbonate. Louie’s blood pH at the time of admission was 6.85 and rose to 7.55 due to the treatment with sodium bicarbonate (Table 8). Treatment with high doses of sodium bicarbonate causes anoxia and brain edema [6, 15-18].
5.3 Louie’s acute bronchopneumonia
The ME described Louie’s lungs as pink to purple and the dorsal aspects of the lungs were much more dense bilaterally than the ventral on palpation. There was slightly irregular purple to pink coloration on transection of the various lung lobes. The weights of the right and left lungs were 89 and 83 g, respectively.
The ME examined the H & E stained sections of the lungs microscopically and stated that Louie had acute bronchopneumonia of the right lung. The left lung was without inflammation. There were wispy eosinophilic residuals of edema fluid.
The ME’s findings in Louie’s lungs on December 20th do not represent the condition of his lungs on December 13th. A chest CT scan exam and a blood test performed on December 13th showed that Louie had lung disease in both lungs and severe lymphocytosis (Tables 6, 7). Louie’s white blood cells and lymphocyte count was 6 times the average normal value and reduced by 90-95% within 6 hours of the treatment with antibiotics. Louie’s treatment with antibiotics in the hospital had led to resolving the pneumonia in his left lung.
5.4 Examination of Louie’s thymus and spleen
The ME described Louie’s thymus as flabby and medium pink without petechiae. The thymus weight was 17 g. The microscopic examination of the H & E stained section of the thymus revealed mildly reduced thickness of the cortex.
Louie’s spleen had a thin, intact capsule tensely distended by red internal tissue within which mild white pulp granules can be identified. The weight of the spleen was 36 g.
The microscopic examination of the H & E stained section of the spleen revealed acute congestion.
5.5 Examination of Louie’s peritoneal cavity and liver
The ME stated that Louie’s peritoneal cavity contained at least 50 mL of yellow fluid over smooth membranes without fibrinous or fibrous adhesion. Louie’s liver weighted 277 g. The microscopic examination of the H & E stained section of Louie’s liver revealed acute sinusoidal congestion.
5.6 Examination of Louie’s other organs
The ME examined the following organs grossly and microscopically in Louie’s case and did not observe any abnormality or sign of trauma: Heart, small intestine and colon, pancreas, kidneys, adrenal gland, urinary bladder, and bone marrow. The weight of Louie’s heart was 39 g. The weights of his right and left kidneys were 24 and 26 g, respectively.
6. The likely causes of Louie’s pneumonia, metabolic acidosis, and respiratory arrest
Louie suffered from respiratory arrest on December 13, 2006 and blood came out of his nose. His father called 911 and the EMS arrived at the seen at 1558. The paramedics used a portable suction unit with catheter and sucked out clear secretions. The tip of the suction catheter had bright red blood on it. They initiated bag-valve mask ventilation and placed Louie on oxygen. His heart rate was 200 beats/minute and had tachycardia.
Louie was taken to the Providence Medical Center’s ER and arrived at 1613. His temperature was 35.2 oC. Examination revealed that his head was normocephalic and atramatic. He was given 0.65 mL of epinephrine (1/1000) subcutaneously and treated with antibiotic, sedative, and anti-seizure medications.
Louie was intubated at 1637 on December 13th and transferred to Children Mercy’s Hospital (CMH). He was pronounced brain dead on December 17th and remained on continuous mechanical ventilation until the life support was pulled off at 1436 on 12/19/2006. An autopsy was performed on December 20th.
The clinical data and medical studies described below indicate that Louie was suffering from acute respiratory infections and septicemia that was caused by Bordetella pertussis. His infections led to weight loss, vitamin K deficiency, pneumonia, bleeding, metabolic acidosis, brain edema, coma, and respiratory arrest. In addition, Louie’s treatment with high doses of epinephrine and sodium bicarbonate caused bleeding and edema.
1) Louie suffered from lymphocytosis and leukocytosis and acute infections with
Bordetella pertussis causes lymphocytosis and leukocytosis in children. For example, McCarthy and Carlile evaluated a 9-month-old white female infant presented with a paroxysmal cough and a white blood cell count of 114 x 103/µL. Her nasopharyngeal culture tested positive for Bordetella pertussis. Her hyperleukocytosis caused by pertussis toxins [13].
A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that he had a white blood cell count of 79.5 x 103/µL and a lymphocyte count of 44.0 x 103/µL. His lymphocyte count was 6 times the average normal level and he was suffering from severe lymphocytosis. Louie was started on Ceftriaxone (antibiotic) IV infusion and his total white blood cells and lymphocyte counts reduced by 90% and 95% within 6 hours, respectively (Table 6).
2) B. pertussis causes pneumonia and pulmonary hemorrhage in children. Fauci et al. stated that the major respiratory complication of pertussis is pneumonia and it is more common in infants (incidence, 21 percent). B. pertussis produces a variety of toxins that impair local defenses, inhibit phagocytosis, and cause local tissue damage and bleeding [6].
Chest CT scan and X-ray exams performed within 2 hours following Louie’s admission to the hospital showed that Louie had pulmonary hemorrhage, pulmonary edema, and lung disease (Table 7). Lin et al. analyzed the clinical presentations of laboratory-confirmed B. pertussis infection of 46 cases (ages: 24 days to 37 years, with a mean of 4.3 years). Leukocytosis (white blood cells > or = 15 x 103/µL) and lymphocytosis (lymphocytes > or = 10 x 103/µL) were observed in 17 and 16 of the individuals, respectively. Fourteen individuals (30.4%) developed complications, among which pneumonia was the most common (92.3%) among infants < or = 1 year of age [10].
3) Infection with B. pertussis causes severe hypoxia and metabolic acidosis.
McCarthy and Carlile stated that B. pertussis causes hyperleukocytosis in infant and hyperleukocytosis with pulmonary leukostasis can result in significant hypoxemia [13]. Louie’s blood pH was 6.85 and suffered from a severe metabolic acidosis. Metabolic acidosis causes brain edema and coma. He was treated with sodium bicarbonate IV and his blood pH rose to 7.55 within 4 days (Table 8).
4) Louie developed apnea, cynosis, tachycardia, sunconjunctival hemorrhage, respiratory failure, and seizure on December 13th and these symptoms have been reported in infants with pertussis. Fauci et al. stated that episodes of cynosis and apnea are common among infants and small children suffering from pertussis (prevalence, 20 to 50 percent). In addition, B. pertussis infection in infants causes sunconjunctival hemorrhage and upper torso petechiae due to increased intrathoracic pressure [6].
Pilorget et al. reported a case of a 40-day-old infant admitted in the intensive care unit with symptoms of bronchiolitis along with a 200 bpm permanent tachycardia. He also had leukocytosis and lymphocytosis. On the second day, convulsions and coma occurred, followed rapidly by respiratory failure, with a subsequent deterioration due to the development of severe pulmonary hypertension. Circulatory failure caused the infant’s death on the beginning of the 5th day. Pertussis was confirmed by PCR on nasopharyngeal swab [11].
5) A head CT scan taken at 2 hours following Louie’s admission to the hospital showed that he had encephalopathy. Acute infection with B. pertussis has caused neurologic complications in infants that include encephalopathy (0.7%) and seizures (2 percent). The potential mechanisms of pertussis-associated encephalopathy were postulated to include hypoxia and/or hypoglycemia due to pertussis toxin, hemorrhages secondary to increased venous pressure, direct neurotoxic effects, and coinfection by neurotoxic virus [6].
6) Malnutrition and weight loss are among the complications of infections with B. pertussis in infants [6]. Louie’s weight on December 11th and December 13th was 7000 and 6800 g, respectively. He had lost 200 g in 2 days. Louie’s weight gain rate between November 28th and December 11th was 73.1 g/day (Table 5).
It seems that Louie’s treating physicians and the medical examiner overlooked the characteristic symptoms and complications of B. pertussis infections described above and in Section 6.1 below. In addition, they did not utilize the standard clinical tests described in Section 6.2 that are used to diagnose B. pertussis infections in children.
6.1 Symptoms and complications of B. pertussis infections in children
B. pertussis colonizes the respiratory tract by adhering to the ciliated epithelial cells and grows to high numbers. It produces a variety of toxins that impair local defenses, inhibit phagocytosis, and cause local tissue damage and hemorrhage. The dramatic inspiratory whoop following a paroxysmal cough is frequently lacking in infants and fever is absent or low [6].
For example, Bocka reported a case of a one-month-old female with a one-week history of low-grade fever and rhinorrhea, and one day of intermittent cough and cyanosis [19]. In addition, Christie and Baltimore reported three cases of neonates who developed pertussis and presented to the hospital with coughing and choking spells without a characteristic inspiratory whoop. Two neonates had apnea, bradycardia, cyanosis, and unresponsiveness. They required prolonged ventilatory support and hospitalization. The other neonate had a terminal pulmonary hemorrhage [20].
B. Pertussis produces lymphocytosis-promoting factor and an absolute lymphocytosis is a characteristic feature in infants. Typically, the total white blood cell count ranges from 10,000 to 30,000 cells/µL, with 50 to 75 percent lymphocytes [6].
An absolute lymphocytosis should provide clue to the diagnosis, particularly in children. Louie’s white blood cells and lymphocytes counts at 17 minutes following admission to the hospital were 79.5 x 103/µL and 44.0 x 103/µL, respectively (Table 6). However, the treating physicians and the medical examiner overlooked this important sign.
Hodge et al. conducted immunophenotyping study using multiparameter flow cytometry and characterized leucocytes from a group of 11 infants (aged 3-6 months) with proven pertussis and from uninfected control subjects. Infants with pertussis showed
an increase in absolute numbers of neutrophils, monocytes, T lymphocytes (both
CD4 and CD8), B lymphocytes (including CD10+/CD19+ hematogones) and natural
killer (NK) cells. There was also an increase in CD45RA+/CD45RO+/CD4+ cells (activated) and CD62L-/CD45RO+/CD4+ cells (Th1-like) [7].
Vaessen et al. stated that babies with respiratory disease and significant lymphocytosis should be considered to have pertussis until proven otherwise. The onset of severe pulmonary hypertension during B pertussis pneumonia is frequently rapid and relentless [12].
In addition, Pilorget et al. 2003 reported that malignant pertussis in very young age of patients characterized by tachycardia, dyspnea with early respiratory failure, frequent neurological symptoms, severe hyperleukocytosis and hyperlymphocytosis, and hyponatremia with oliguria and edema. Mortality is more than 75% despite the various treatments and life support measures that have been attempted [11].
Furthermore, Ferrer Marcellés et al. performed a retrospective study in Barcelona, Spain, involving 161 patients with culture positive Bordetella spp, aged less than 18 years. Of
These children, complete information was available in 149 (79 boys and 70 girls) with a median age of 3 months (range: 13 days-17 years); 77.2 % were aged 6 months or
less. Bordetella spp. was associated with other bacteria in 28.2 % of the patients, viruses in 13.4 % and a bacterium and a virus in 4.7 %.
One hundred twenty-one children required hospitalization, of which 14.9 % were
admitted to the intensive care unit. Age was the only factor associated with risk
for hospitalization, which was more frequent in younger infants (p < 0.0001).
Paroxysmal cough with cyanosis was present in 53.4 % of the patients,
leucocytosis with lymphocytosis occurred in 67.5 % and apneas were present in
21.5 %.
Chest X-ray revealed atelectasis in 34.1 %. The mean length of hospital stay was 11 days (range: 1-70 days). Three boys aged less than 3 months with malignant pertussis syndrome died (lethality: 2 %) [22].
Francis Centeno et al. also conducting a retrospective study based on 144 cases of children with a clinical diagnosis of whooping cough. The age varied between 20 days
and 30 months. The initial symptoms were: paroxysmal cough in 44 (95.7%), cyanosis in 34 (73.9%), inspiratory whoop in 27 (58.7%), respiratory distress in 12 (26.1%) and post-tussive vomiting in 10 (21.7%). Nasopharyngeal specimens for culture were taken in 119 cases. Cultures were positive for B. pertussis in 46 patients (38.7%). Leukocytosis (mean: 19.82 x 103/µL) and lymphocytosis (mean: 13.05 x 103/µL) were common findings [23].
6.2 Procedures used to diagnose B. pertussis infections in children
In Louie’s case, the treating physicians and the medical examiner did not utilize the following standard diagnosed procedures that are used to diagnose B. pertussis infections in children.
1) B. pertussis is a small, nomotile, gram-negative coccobacillus that is slow growing and fastidious in its growth requirements. The standard diagnostic test used is the isolation of B. pertussis from nasopharyngeal swab culture. A calcium alginate swab is inserted into the nares and maintained in contact with the nasopharynx for at least 10 seconds to permit moistening. Then, the swab placed in to transport medium such as Regan-Lowe charcoal medium or plated directly onto fresh Bordet-Gengou agar or another suitable agar.
A nasopharyngeal aspirate collected with a syringe attached to a fine plastic catheter is also used as a suitable alternative specimen. Growth typically requires 3 to 5 days of incubation at 36 oC. Suspicious colonies usually identified by direct fluorescent antibody staining or agglutination [6, 9].
Finger et al. investigated a total of 2,881 clinically diagnosed cases of children with whooping cough. The children had a mean age of 4.1 years and 11% of the children were younger than one year. In relation to clinical symptoms, the isolation rate of Bordetella pertussis or Bordetella parapertussis from nasopharyngeal swabs continuously decreased with the duration of paroxysms, starting with 56% positive swabs on day 1 [21].
In addition, Nakamura et al. reported a case of a 28-day-old female infant with pertussis presented with severe acute bronchiolitis and cyanosis. He also had apneic episodes and peripheral lymphocytosis. The presence of Bordetella pertussis in nasopharyngeal and intratracheal aspirates was examined using loop-mediated isothermal amplification (LAMP) based on the insertion sequence IS481. LAMP of the nasopharyngeal and intratracheal aspirates was positive for B. pertussis and a diagnosis of neonatal pertussis was made [24].
2) Measuring antibodies in serum: Deen et al. evaluated household contacts of primary pertussis cases. Infection was determined by culture, direct fluorescent antibody assay, and serological criteria. Agglutinin titers and values of ELISA IgG and IgA antibodies to
lymphocytosis-promoting factor, filamentous hemagglutinin, and pertactin were
determined [25].
In addition, Bollag and Matter investigated 3 cases of children meeting the criteria of infection with pertussis. The clinical diagnosis was confirmed by the detection of elevated serum IgA to filamentous hemagglutinin (FHA) [26].
7. The likely causes of Louie’s intracranial, retinal, and pulmonary bleeding and brain edema
Louie was admitted to the hospital on December 13, 2006 and died on December 19th. Dr. Erik Krag Mitchell performed the autopsy on Louie’s body on December 20th. External examination did not reveal any evidence of injury caused by trauma. In addition, an X-ray exam of Louie’s body performed in the morgue did not show broken bone or injuries caused by trauma [14].
Dr. Mitchell examined Louie’s brain and meninges grossly and stated that Louie had diffuse engorgement of the meninges with the films of subarachnoid and subdural blood in the anterior fossa and over the vertex. There was also blood in the posterior fossa. In addition, an eye exam conducted on December 14th revealed that Louie had diffuse retinal hemorrhage (old and new) in both eyes.
The medical examiner (ME) alleged that vigorous shaking of the head caused Louie’s intracranial and retinal bleeding and brain edema [3, 4, 14]. The clinical data and pertinent medical studies described below and the previous sections of this report indicate that the ME’s allegations are not supported by medical facts.
1) Louie’s intracranial and retinal bleeding caused by bacterial infection, vitamin K deficiency, liver injury, and the treatment with high dose of epinephrine (0.65 mL, SQ) received on December 13th.
2) Louie developed subarachnoid bleeding following his admission to the hospital on December 13th. Subarachnoid bleeding observed at autopsy was not shown on the head CT scan performed at 2 hours following Louie’s admission to the hospital.
3) Louie’s bleeding was not limited to the intracranial and retinal bleeding. He also had pulmonary bleeding, which was caused by Bordetella pertussis.
4) Louie developed Disseminated Intravascular Coagulation (DIC) after his admission to the hospital, which caused bleeding.
5) Louie developed severe brain edema and edema in other tissues during his 7 days hospitalization as a result of anoxia, treatment with high doses of sodium bicarbonate, and kidney problems.
6) Increased intracranial pressure contributed to Louie’s retinal bleeding.
7.1 Bordetella pertussis, liver damage, and DIC caused bleeding in Louie’s case
Louie suffered from acute B. pertussis infections and septicemia, which caused weight loss, vitamin K deficiency, pneumonia, metabolic acidosis, and injuries in organs. The increased level of alkaline phosphatase (AlkP) and glutamic oxaloacetic transaminase (GOT) in serum indicates that Louie suffered from acute liver injury. A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that his ALK and SGOT levels in serum were equal to 161% and 323% of the upper normal level value, respectively (Table 11).
The liver plays a central role in the clotting process. Injuries and diseases of the liver are usually associated with coagulation disorders due to multiple processes. These include reducing the synthesis of clotting and inhibitor factors, decreasing the clearance of activated factors, and producing quantitative and qualitative platelet defects. Some of these abnormalities may lead to hyperfibrinolysis and the acceleration of the intravascular coagulation process [27-33].
A blood analysis performed at 2 hours following Louie’s admission to the hospital revealed that he had elevated prothrombin time (PT) and partial thromboplastin time (PTT). He had a PT of 20.8 seconds (normal: 12.4-15.4) and a PTT of 46.4 seconds (normal: 24.5-37.5). In addition, his PTT reached a very high level of 80.2 seconds on December 16th. PT measures clotting factors II, V, VII, X and fibrinogen and these factors are synthesized in the liver.
Furthermore, Louie suffered from acute bacterial infections as indicated by the highly elevated white blood cells and lymphocytes counts. A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that he had a white blood cell count of 79.5 x 103/µL and a lymphocyte count of 44.0 x 103/µL. His lymphocyte count was 6 times the average normal level and he was suffering from severe lymphocytosis. Louie was started on Ceftriaxone (antibiotic) IV infusion and his total white blood cells and lymphocyte counts reduced by 90% and 95% within 6 hours, respectively (Table 6).
Septicemia is frequently accompanied by changes in the plasmatic as well as cellular coagulation systems and by microclot formation. The activation of coagulation by endotoxin is mediated by synthesis of tissue factor by monocytes and endothelial cells.
Some microorganisms have specific properties, which affect individual components of hemostasis and thus increase their virulence. Furthermore, thrombocytopenia, thrombocytopathy and endothelial cell damage caused by a direct effect of the toxic agents contribute to the bleeding diathesis [34, 35].
A blood analysis performed at 17 minutes following Louie’s admission to the hospital revealed that his hematological values were within the normal range. However, his blood analysis performed at about 2 hours following admission indicates that he developed anemia. His RBC and hemoglobin level reduced by 34% (Tables 13, 14).
Furthermore, Louie received RBC transfusions prior to 0030 on December 14th and his RBC, hematocrit value, and hemoglobin level increased and reached a normal level. However, his blood analysis performed at 1540 on December 15th showed that he developed anemia due to bleeding (Table 13). A significant amount of blood was detected in his urine (Table 15).
Louie suffered from coagulation problem and DIC. His level of D-Dimer was more than 4 times the normal level and his platelet count reduced by 63% within 6 hours (Tables 12, 13). Levi stated that DIC occurs frequently in septic patients and associated with increased mortality. Organ dysfunction is also common sequelae that is strongly correlated with DIC. Cytokines released early in the course of sepsis stimulate a procoagulant state that causes development of intravascular fibrin deposition. In a later stage of DIC, bleeding may occur in parallel because of consumption of clotting factors and inhibitors [36].
Voves et al. evaluated the score for DIC published by the International Society for Thrombosis and Haemostasis (ISTH) in a 32 patients suffering from severe sepsis and 8 patients with septic shock. Fibrin monomer and D-dimer were chosen as fibrin-related markers (FRM), respectively. DIC scores for non-survivors (n = 13) as well as for septic shock patients were higher (P < 0.04) compared with survivors and patients with severe sepsis, respectively.
Using fibrin monomer and D-dimer, 30 and 25% of patients suffered from overt DIC. Overt DIC was associated with significantly elevated thrombin-antithrombin complexes and plasminogen activator inhibitor type-1 levels as well as with significantly lower factor VII clotting activity. Patients with overt DIC had a significantly higher risk of death and of developing septic shock. Since more than 95% of the sepsis patients had elevated FRM, the DIC score was strongly dependent on prolongation of the prothrombin time and platelet counts [37].
Furthermore, Gando et al. evaluated 19 patients with the diagnosis of severe sepsis or septic shock and 9 control patients to obtain systematic information on the extrinsic coagulation pathway, as well as to investigate the time course of the coagulation abnormalities in sepsis. Tissue factor antigen concentration (tissue factor antigen), prothrombin fragment F1+2, thrombin antithrombin III complex, fibrinopeptide A, D-dimer, and antithrombin III concentrations were measured on the day of diagnosis of severe sepsis and septic shock, and on days 1, 2, 3, and 4 after diagnosis [38].
They found that the concentrations of tissue factor antigen, prothrombin fragment F1+2, fibrinopeptide A, and D-dimer were significantly increased in patients with severe sepsis and septic shock compared with control subjects. Significantly, low antithrombin III concentrations were observed in the septic patient groups compared with control subjects. Significant correlations were noted between tissue factor antigen and the Disseminated Intravascular Coagulation score (r2=.236, p< .0001) and the number of dysfunctioning organs (r2=.229, p=.035) [38].
7.2 Louie’s risks for vitamin K deficiency and intracranial bleeding
Vitamin K controls the formation of coagulation factors II (prothrombin), VII (proconvertin), IX (Christmas factor), and X (Stuart factor) in the liver. Other coagulation factors that depend on vitamin K are proteins C, S, and Z. These vitamin K-dependent proteins contain the amino acid -carboxyglutamic acid and the carboxyl groups of the glutamic acid residues that provide the vitamin-K-dependent proteins with characteristic calcium and phospholipid binding properties. Vitamin K deficiency has led to the production of abnormal vitamin K-dependent factors, which lack gamma-carboxy glutamic acid residues in the NH2-terminal part of their molecules [17, 18, 39-44].
In humans, the body does not synthesize the 1, 4 naphthoquinone nucleus of vitamin K and gets it from food. In addition, the bacteria in the intestinal tract synthesize vitamin K and can supply part of the vitamin K requirement. Signification reduction of food intake occurred in serious illness can lead to vitamin K deficiency and intracranial bleeding in children [39, 41, 45-48].
Louie was suffering from bacterial infections and did not eat well. His weight on December 11th and December 13th was 7000 and 6800 g, respectively and had lost 200 g in 2 days. Louie’s weight gain rate between November 28th and December 11th was 73.1 g/day, which is equal to 401% his weight gain rate occurred between September 22nd and November 28th. These data indicate that Louie’s body was retaining fluids.
Louie’s length on November 28th and December 13th was 55.9 and 59 cm, respectively. His length increased at the rate of 6.2 cm/month, which is 200% of his length increase rate observed between July 11th and September 22nd (Table 5). These data indicate that Louie’s body was retaining fluids and he was sick for a significant period of time. His PT and PTT were elevated on December 13th, which indicates that he was suffering from vitamin K deficiency.
Intracranial bleeding and bleeding in other locations have been reported in children suffered from vitamin K deficiency. For example, 1) Nishio et al. examined 84 cases of intracranial hemorrhage in children due to vitamin K deficiency from literatures. Hemorrhage sites were identified by CT scan in these children. Subarachnoidal hemorrhage was in 72 cases (85.7%), subdural hemorrhage was in 41 cases (48.8%), intracerebral hematomas was in 36 cases (42.9%) and intraventricular hemorrhage was in 9 cases (10.7%) [45].
In addition, 2) Demirören et al. described the clinical and laboratory findings of 19 infants with intracranial hemorrhage (ICH) due to vitamin K deficiency. The mean age at onset of the symptoms was 49 +/- 18 days. The localizations of the ICHs were as follows: Parenchymal (47%), subarachnoid (47%), subdural (42%), and intraventricular (26%).
[49].
Furthermore, Choo et al. conducted a retrospective study of 42 newborns admitted to the hospital for spontaneous bleeding. Subdural hemorrhage was the most common form of intracranial haemorrhage, followed by subarachnoid haemorrhage. The six most common presenting clinical features were pallor, jaundice, umbilical cord bleeding, tense fontanelle, convulsions and hepatomegaly. The overall case fatality rate was 14%. None of the infants had bleeding due to inherited coagulopathy or disseminated intravascular coagulation [50].
Chaou et al. also reported 32 cases of infants (0.5-6.0 months of age) who developed intracranial bleeding due to vitamin K deficiency. Computerized tomography showed mild to severe intracranial hemorrhage. Most (90.6%) had subarachnoid hemorrhage, either alone or in combination with subdural hemorrhage (37.5%), parenchymal hemorrhage (31.3%), or intraventricular hemorrhage (12.5%). In three cases (9.4%) the
infratentorial region was involved [51].
In addition, Pooni et al. evaluated 42 infants who developed intracranial hemorrhage (ICH) and bleeding in other sites as a result of vitamin K deficiency. They found that 71% of these infants presented with intracranial hemorrhage, the most common site was intracerebral and multiple ICH. Visible external bleeding was noted in 1/3rd of the infants. Three infants died [52].
Furthermore, Aydinli et al. conducted a retrospective study included 11 babies between 30 and 119 days of age, who developed bleeding due to vitamin K deficiency. The localizations of the intracranial hemorrhage were as follows: intracerebral (91%), subarachnoid (46%), subdural (27%), and intraventricular (27%) [53].
I have also evaluated the medical records of several babies who were ill and developed intracranial bleeding. Differential diagnosis identified vitamin K deficiency as the primary cause of the bleeding in these children [17, 18, 41-44].
7.3 Louie’s dose of epinephrine causes intracranial bleeding
Louie was admitted to the hospital at 1613 on December 13th and was given 0.65 mL of epinephrine by subcutaneous route. Intracranial bleeding has been reported in some children and adults treated with high therapeutic doses of epinephrine [16-18, 54-57]. For example, bleeding (intracerebral, subdural and/or subarachnoid hemorrhage) was reported as one of the serious adverse reactions of epinephrine, even when given to individuals at a low dosage level of 0.05 mg subcutaneously [54].
In addition, Horowitz et al. reported the development of acute cardiac arrest and fatal subarachnoid hemorrhage in an individual who suffered from allergic reaction and treated with epinephrine subcutaneously [55]. I have also evaluated medical records and other medical evidence involving four cases of children who suffered from cardiac arrest and treated with high doses of epinephrine. These children had intracranial bleeding and differential diagnosis identified epinephrine as important factor in causing bleeding in these children [16-18, 57].
7.4 Louie’s brain edema and fluid retention developed following admission
Louie developed brain edema and edema in other locations following his admission to the hospital. Anoxia, septicemia, metabolic problems, treatment with high doses of sodium bicarbonate, and kidney problems caused his severe edema. The following clinical data describe the progress, severity of Louie’s edema, and the changes in the pH due to metabolic acidosis and the treatment with high doses of sodium bicarbonate.
1) The medical examiner (ME) reported that Louie’s calvarial sutures were widely separated up to a maximum of approximately 1 inch and bulge. Louie’s brain was very soft due to edema. A head CT scan exam performed following Louie’s admission on December 13th did not show that he had separation of the cranial sutures (Table 16). The separation of cranial sutures occurred in the hospital due to increased intracranial pressure caused by edema. Louie’s head circumference (HC) on December 14th and at autopsy was 42 and 45 cm, respectively.
2) Louie’s length on December 14th and at autopsy was 59 and 65 cm, respectively. His length increased by 6 cm during 6 days of hospitalization. In addition, Louie’s weight on December 13th and at autopsy was 6800 and 8190 g, respectively. His weight increased by 20.44% during his 7-day hospitalization. These data indicate that Louie developed severe body edema following his admission to hospital on December 13th.
The ME stated that Louie’s body was fairly obviously edematous with flat pressure reduction of edema on the right side of the back of the head. His eyelids were swollen and edematous to the point that his conjunctivae cannot be adequately examined.
3) Louie’s blood pH at the time of admission was 6.85 and rose to 7.55 due to the treatment with sodium bicarbonate (Table 8). Treatment with high doses of sodium bicarbonate causes anoxia and brain edema [6, 15-18].
7.5 Increased intracranial pressure causes retinal bleeding
Louie’s intracranial pressure was highly increased following his admission to the hospital as indicated by the degree of the calvarial sutures separation and the change in Louie’s HC. His HC increased by at least 3 cm following admission. A sudden rise in the ICP has caused intraocular bleeding in some individuals.
For example, Medele et al. performed prospective ophthalmological examination in 22 consecutive individuals with subarachnoid hemorrhage (SAH) or severe brain injury and elevated ICP. Thirteen individuals were admitted to the hospital for SAH and nine for severe brain injury. Monitoring of ICP was performed at the time of admission via a
ventricular catheter. Initial ICP exceeded 20 mm Hg in all individuals.
Indirect ophthalmoscopy without induced mydriasis was performed within the 1st week after the acute event. Retinal or vitreous hemorrhage was seen in six (46%) of 13
individuals with SAH and in four (44%) of nine individuals with severe brain injury.
Ocular bleeding was found bilaterally in three individuals with SAH and in one
individual with severe brain injury (18%) [58].
Furthermore, Stiebel-Kalish et al. evaluated the medical records of 70 individuals with subarachnoid hemorrhage resulted from ruptured cerebral aneurysms. They found that
30 eyes of 19 individuals had intraocular hemorrhages; 14 eyes had a vitreous hemorrhage; 12 eyes had subhyaloid blood without a vitreous hemorrhage; and 4 eyes had retinal hemorrhages alone [59].
8. Conclusions
Louie Rodriguez suffered from a respiratory arrest on December 13, 2006 and his father called 911. Louie was hospitalized and died on December 19th. An autopsy was performed on Louie’s body on December 20th. The medical examiner (ME) and Louie’s treating physicians alleged that violent shaking of the head (Shaken Baby syndrome) caused Louie’s injuries and death. Louie’s father was accused of killing Louie and arrested. My investigation in this case reveals the following:
1) Louie suffered from acute infections with Bordetella pertussis, which caused weight loss, vitamin K deficiency, pneumonia, bleeding, metabolic acidosis, brain edema, coma, and respiratory arrest.
2) Louie’s treating physicians and the ME did not use the standard diagnosed procedures that are used to diagnose B. pertussis infections in children. B. pertussis should be isolated from Louie’s nasopharyngeal region using calcium alginate swab. The swab then placed into transport medium (Regan-Lowe charcoal medium) or plated directly onto fresh Bordet-Gengou agar. Growth typically requires 3 to 5 days of incubation at 36 oC. Colonies usually identified by direct fluorescent antibody staining or agglutination.
3) Louie’s intracranial and retinal bleeding was caused by B. pertussis infection and septicemia, vitamin K deficiency, liver damage, pneumonia, and his treatment with high doses of epinephrine. Increased intracranial pressure contributed to the retinal bleeding.
4) Louie developed brain severe edema and edema in other locations following his admission to the hospital as a result of anoxia, metabolic and kidney problems, and treatment with sodium bicarbonate.
5) The allegations given that vigorous shaking of the head caused Louie’s injuries and death are not supported by medical facts. Louie’s illness and death were caused by acute infections with B. pertussis and septicemia.
References
[1] Louie Rodriguez’s medical records. St. Joseph’s Hospital, Tucson, Arizona July 11-August 4th, 2006.
[2] Louie Rodriguez’s medical records. Providence Medical Center. 8929 Parallel
Parkway. Kansans City, KS 66112. September 22-Dember 13, 2006.
[3 ] Court Transcript. State of Kansas (Plaintiff) vs. Jaime Rodriguez (Defendant). In the District Court of Wyandotte County, Kansas Criminal Department (No. 06CR 2332), April 4, 2007.
[4] Court Transcript. State of Kansas (Plaintiff) vs. Jaime Rodriguez (Defendant). In the District Court of Wyandotte County, Kansas Criminal Department (No. 06CR 2332), January 8-11, 2008.
[5] Louie Rodriguez’s medical records. Children’s Mercy Hospital. Kansans City, KS
66112. December 13-19, 2006.
[6] Fauci AS, Braunwald E, Isslbacher KJ, Wilson, JD, Martin JB, Kasper DL, Hauser
SL, and Longo DL. Harrison’s Principles of Internal Medicine. McGraw-Hill Companies, Inc. New York USA, ed. 14, 1998.
[7] Hodge G, Hodge S, Markus C, Lawrence A, Han P. A marked decrease in L-selectin expression by leucocytes in infants with Bordetella pertussis infection: leucocytosis explained? Respirology. 2003 Jun; 8(2):157-62.
[8] Heininger U, Stehr K, Schmitt-Grohé S, Lorenz C, Rost R, Christenson PD, Uberall M, Cherry JD. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr Infect Dis J. 1994 Apr; 13 (4):306-9.
[9] Heininger U, Klich K, Stehr K, Cherry JD. Clinical findings in Bordetella pertussis
infections: results of a prospective multicenter surveillance study. Pediatrics. 1997 Dec;100(6):E10.
[10] Lin PY, Chiu CH, Wang YH, Su LH, Chia JH, Huang YC, Chung PW, Wu TL, Lin TY. Bordetella pertussis infection in northern Taiwan, 1997-2001. J Microbiol
Immunol Infect. 2004 Oct; 37(5):288-94.
[11] Pilorget H, Montbrun A, Attali T, Tiran-Rajaofera I, Bony C, Brayer C, Sampériz
S, Alessandri JL. Malignant pertussis in the young infant. Arch Pediatr. 2003
Sep;10(9):787-90.
[12] Vaessen S, Anthopoulou A, Bricteux G. Clinical case of the month. Fatal pertussis infection in a 2 month old infant. Rev Med Liege. 2006 Mar; 61(3):145-8.
[13] McCarthy VP, Carlile JR. Hyperleukocytosis with pertussis. J Assoc Acad Minor
Phys. 1997; 8(3):52-4.
[14] Autopsy report in the case of Louie Rodriguez (L06-12-102 WY) singed by Dr. Erik K. Mitchell on February 23, 2007.Wyandotte County, Kansas.
[15] Bureau MA, Begin R, Berthiaume Y, Shapcott D, Khoury K, and Gagnon N.
Cerebral hypoxia from bicarbonate infusion in diabetic acidosis. Journal of Pediatrics; 96:968-73, 1980.
[16] Al-Bayati MA. Analysis of causes that led to Toddler Alexa Shearer’s cardiac arrest and death in November 1999. Medical Veritas; Vol. 1(1): 86-117, 2004. infusion
[17] Al-Bayati MA. Analysis of Causes That Led to Baby Lucas Alejandro Mullenax-Mendez’s Cardiac Arrest and Death in August-September of 2002. Medical Veritas; Vol. 1 (1): 45-63, 2004. infusion
[18] Al-Bayati MA. Analysis of causes that led to subdural bleeding, skull and rib
fractures, and death in the case of baby Averial Buie. Medical Veritas; Vol. 4 (2):
pages 1452-69, 2007.
[19] Bocka JJ. Pertussis in an infant. J Emerg Med. 1989 Jul-Aug; 7(4):345-8.
[20] Christie CD, Baltimore RS. Pertussis in neonates. Am J Dis Child. 1989
Oct; 143(10):1199-202.
[21] Finger H, Wirsing von König CH, Tacken A, Wassilak SG. The epidemiological
situation of pertussis in the Federal Republic of Germany. Dev Biol Stand. 1991; 73:343-55.
[22] Ferrer Marcellés A, Moraga Llop FA, Olsina Tebar M, Campins Martí M, Planells
Romeu I. Culture-confirmed whooping cough in a tertiary center over a twelve-year period. An Pediatr (Barc). 2003 Apr; 58(4):309-15.
[23] Francis Centeno M, Borque Andrés C, del Castillo Martín F, Díez Sebastián J,
García Hortelano J. Whooping cough: a retrospective study of the cases diagnosed
over a period of 15 years. An Esp Pediatr. 1998 Sep; 49(3):280-3.
[24] Nakamura A, Sakano T, Nakayama T, Shimoda H, Okada Y, Hanayama R, Nomoto K, Suto T, Kinoshita Y, Furue T, Ono H, Ohta T. Neonatal pertussis presenting as acute bronchiolitis: direct detection of the Bordetella pertussis genome using loop-mediated isothermal amplification. Eur J Pediatr. 2009 Mar;168 (3):347-9.
[25] Deen JL, Mink CA, Cherry JD, Christenson PD, Pineda EF, Lewis K, Blumberg
DA, Ross LA. Household contact study of Bordetella pertussis infections. Clin Infect
Dis. 1995 Nov; 21 (5):1211-9.
[26] Bollag U, Matter L. Pertussis in clinical practice–critical evaluation of diagnosis and Epidemiology. Schweiz Med Wochenschr. 1992 Oct 10;122 (41):1530-5.
[27] Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis. 2002 Feb; 22 (1):83-96.
[28] Denninger MH. Liver diseases and hemostasis. Pathol Biol (Paris). 1999 Nov; 47 (9):1006-15.
[29] Mammen EF. Coagulation abnormalities in liver disease. Hematol Oncol Clin North Am. 1992 Dec; 6 (6):1247-57.
[30] Papadopoulos V, Filippou D, Manolis E, Mimidis K. Haemostasis impairment in
patients with obstructive jaundice. J Gastrointestin Liver Dis. 2007 Jun; 16 (2):177-86.
[31] Peck-Radosavljevic M. Review article: coagulation disorders in chronic liver
disease. Aliment Pharmacol Ther. 2007 Nov; 26 Suppl 1:21-8.
[32] Trotter JF. Coagulation abnormalities in patients who have liver disease. Clin Liver Dis. 2006 Aug;10 (3):665-78.
[33] Téllez-Avila FI, Chávez-Tapia NC, Torre-Delgadillo A. Coagulation disorders in
cirrhosis. Rev Invest Clin. 2007 Mar-Apr; 59 (2):153-60.
[34] Müller-Berghaus G. Sepsis and blood coagulation. Behring Inst Mitt. 1986 Feb; (79):131-41.
[35] Hudecek J, Paceková M, Chudej J, Kubisz P. Infection and hemostasis. Vnitr Lek. 2004 Jun; 50 (6):453-61.
[36] Levi M. Pathogenesis and treatment of disseminated intravascular coagulation in
the septic patient. J Crit Care. 2001 Dec;16 (4):167-77.
[37] Voves C, Wuillemin WA, Zeerleder S. International Society on Thrombosis and
Haemostasis score for overt disseminated intravascular coagulation predicts organ
dysfunction and fatality in sepsis patients. Blood Coagul Fibrinolysis. 2006 Sep; 17 (6):445-51.
[38] Gando S, Nanzaki S, Sasaki S, Aoi K, Kemmotsu O. Activation of the extrinsic
coagulation pathway in patients with severe sepsis and septic shock. Crit Care Med.
1998 Dec; 26 (12):2005-9.
[39] The Merck Manual of Diagnosis and Therapy. Editors Beets MH and Berkow R,
Seventeenth edition, 1999. Published by Merck Research Laboratories, Whitehouse
Station, N.J.
[40] Thorp JA, Gaston L, Caspers DR, Pal ML. Current concepts and controversies in
the use of vitamin K. Drugs. 1995 Mar; 49 (3):376-87.
[41] Al-Bayati MA. Analysis of causes that led to bleeding, cardiac arrest,
and death in the case of baby Nadine. Medical Veritas; Vol. 3 (2):997-1012, 2006.
[42] Al-Bayati MA. Analysis of causes that led to subdural bleeding and rib fractures in the case of baby Patrick Gorman. Medical Veritas; Vol. 3 (2):1019-1040, 2006.
[43] Al-Bayati MA. Analysis of causes that led to rib and skull fractures and intracranial bleeding in the case of the premature triplets Parneet, Sukhsaihaj, and Imaan. Medical Veritas; Vol. 5 (1):1589-1609, 2008.
[44] Al-Bayati MA. Analysis of causes that led to baby Ryan’s hemorrhagic pneumonia, cardiac arrest, intracranial bleeding, and retinal bleeding. Medical
Veritas; Vol. 5 (2), 1757-74, 2008.
[45] Nishio T, Nohara R, Aoki S, Sai HS, Izumi H, Miyoshi K, Morikawa Y, Mizuta R.Intracranial hemorrhage in infancy due to vitamin K deficiency: report of a case
with multiple intracerebral hematomas with ring-like high density figures. No To
Shinkei. 1987 Jan; 39 (1):65-70.
[46] Bhat RV, Deshmukh CT. A study of Vitamin K status in children on prolonged
antibiotic therapy. Indian Pediatr. 2003 Jan; 40 (1):36-40.
[47] Sunakawa K, Akita H, Iwata S, Sato Y. Clinical superinfection and its attendant
symptomatic changes in pediatrics. Infection. 1985;13 Suppl 1:S103-11.
[48] de Montalembert M, Lenoir G, Saint-Raymond A, Rey J, Lefrere JJ. Increased
PIVKA-II concentrations in patients with cystic fibrosis. J Clin Pathol. 1992 Feb; 45 (2):180-1.
[49] Demirören K, Yavuz H, Cam L. Intracranial hemorrhage due to vitamin K
deficiency after the newborn period. Pediatr Hematol Oncol. 2004 Oct-Nov; 21 (7):585-92.
[50] Choo KE, Tan KK, Chuah SP, Ariffin WA, Gururaj A. Haemorrhagic disease in
newborn and older infants: a study in hospitalized children in Kelantan, Malaysia.
Ann Trop Paediatr. 14 (3):231-7, 1994.
[51] Chaou WT, Chou ML, Eitzman DV. Intracranial hemorrhage and vitamin K
deficiency in early infancy. J Pediatr. 105 (6):880-4, 1984.
[52] Pooni PA, Singh D, Singh H, Jain BK. Intracranial hemorrhage in late
hemorrhagic disease of the newborn. Indian Pediatr. 2003 Mar; 40 (3):243-8.
[53] Aydinli N, Citak A, Caliskan M, Karabocuoglu M, Baysal S, Ozmen M.
Vitamin K deficiency–late onset intracranial haemorrhage. Eur J Paediatr Neurol
2(4):199-203, 1998.
[54] Goodman & Gilman’s. The Pharmacological Basis of Therapeutics. Editors:
Hardman JG, Limbird LE, Molinoff, PB, Ruddon RW, and Gilman AG. Ninth
Edition, 1996. McGraw-Hill, New York.
[55] Horowitz BZ, Jadallah S, Derlet RW. Fatal intracranial bleeding associated with
prehospital use of epinephrine. Ann Emerg Med. 1996 Dec; 28 (6):725-7.
[56] Know O, Chung S, Lee K, Kim S. Spontaneous subarachnoid hemorrhage after
intravenous epinephrine use for multiple bee stings. Am J Emerg Med. 2007
Feb; 25 (2):249-50.
[57] Al-Bayati MA. Analysis of causes that led to toddler Steven Young’s respiratory
arrest, intracranial and retinal bleeding, bronchopneumonia, peritonitis, and death.
Medical Veritas; Vol. 5 (2), 1775-96, 2008.
[58] Medele RJ, Stummer W, Mueller AJ, Steiger HJ, Reulen HJ. Terson’s syndrome in subarachnoid hemorrhage and severe brain injury accompanied by acutely raised
intracranial pressure. J Neurosurg. 1998 May; 88 (5):851-4.
[59] Stiebel-Kalish H, Turtel LS, Kupersmith MJ. The natural history of
nontraumatic subarachnoid hemorrhage-related intraocular hemorrhages. Retina. 2004 Feb; 24 (1):36-40.
About the Author:
Mohammed Ali Al-Bayati is a toxicologist and pathologist with over 20 years experience in these fields, and has evaluated many cases of children who died suddenly from unexplained causes, including cases of children and adults who suffered from acute and/or chronic illnesses. These causes of illnesses and death required a differential diagnosis. The author has also served as an expert witness in many medical-legal cases involving children and adults. I have published over 50 articles in medical and scientific journals. Author contact: Toxi-Health International, 150 Bloom Drive, Dixon, CA 95620; Phone: (707) 678-4484; Fax: (707) 678-8505